Complement C1q and Its Receptors
The complement system consists of more than 30 proteins, which can be activated through the classical pathway, lectin pathway, and alternative pathway. When complements are activated, complement proteins are cleaved into small fragments that can be recognized by various cell surface complement receptors. The primary function of these cell surface complement receptors is to facilitate the innate immune system's elimination of microorganisms and foreign proteins and cellular debris in the circulatory system.
In 1977, Sobel and colleagues discovered that the complement component C1q binds B cells and other cells in a concentration-dependent and saturated manner, and since then people have gradually found that C1q also has the ability to regulate various cellular responses. As a result, researchers' interest in C1q and its binding proteins or receptors has also increased. Current research results show that C1q's cell surface receptors are quite complex. Some receptors or binding proteins can not only bind C1q, but also other ligands. And the distribution of various receptors or binding proteins in cells is not the same. The C1q binding proteins or receptors currently found include C1qRp, gC1qbp, C1qRO2-, CRT / cC1qR / collectin receptor and CR1.
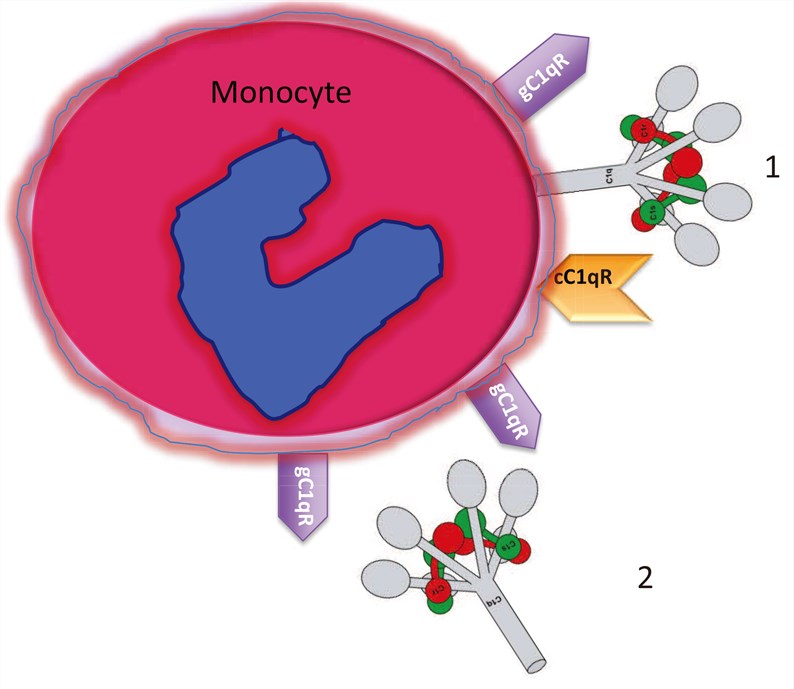
Fig.1 Circulating blood monocytes express C1 and C1qRs. (Ghebrehiwet, 2014)
Role of C1q in The Classical Activation Pathway of Complement
The classical complement activation pathway is initiated by the combination of C1q in the C1 complex with immune complexes (IC) or other non-immune complexes. C1q is a glycoprotein composed of 6 subunits, each of which contains A, B and C peptide chain. The near N-terminal collision-like region (CLR) and the C-terminal global region (GR) are two functional regions of C1q. Among them, CLR is combined with C1r and C1s to form C1qC1r2C1s2. Through the recognition and combination of GR to IC, C1r and C1s are activated, and activated C1r2C1s2 is quickly shed from the CLR of C1q. Free CLR of C1q can bind to C1qRp, C1qRO2-, CR1 and initiate various cellular responses, including phagocytosis, chemotaxis induction, and stimulation of oxidation.
Complement C1q Receptors
-
C1qRp
C1qRp is a binding protein of C1q, which is widely distributed in B lymphocytes and their parental cell lines, but deficient in most tissue macrophages. Its peptide chain is composed of 652 amino acids, including a signal peptide containing 21 amino acids and a mature peptide chain containing 631 amino acids. The N-terminus is located outside the cell, with a serine/ threonine-rich mucin-like region that binds calcium and stabilizes protein-protein interactions. C1qRp can not only bind free C1q, but also can bind MBL, SP-A, and cementin with C1q-like structure, and the binding sites are the same. Studies have found that stimulation of IFN-γ, TNF-α, and LPS can up-regulate the expression of C1qRp, gC1qbp in vascular endothelial cells, and the up-regulation effect is time- and dose-dependent. C1qRp can enhance FcR, C1R-mediated phagocytosis, can induce human umbilical cord endothelial cells to secrete IL-8 and participate in cell adhesion and diffusion.
-
gC1qbp
gC1qbp/ P33 is an acidic glycoprotein with a molecular weight of 3.3 KD and a crystal structure of three P33 molecules forming a hollow ring-shaped trimer structure with uneven charge distribution. gC1qbp can bind to GR of C1q, transcription factor IIB, laminin B receptor, high molecular weight kininogen, factor XII, hyaluronic acid. It can also interact with EBNA-1 of EB virus-1, herpes simplex virus ORFP, adenovirus core protein V, HIV-1 Tat, gp120 and other viral proteins. The study found that almost all mammalian cells have gC1qbp, but only a small amount of gC1qbp on the cell surface, most of which are in the mitochondria in the cytoplasm. The secretion of gC1qbp can regulate the response of many cells and endovascular proteins.
-
C1qRO2-
Poly-C1q can stimulate leukocytes to produce O2-. Cells produce superoxide and other forms of oxidation products, which is a protective mechanism for killing pathogenic microorganisms. C1q-mediated superoxide production cannot be inhibited by G protein inhibitors, and the production of this superoxide does not require stable cell-to-cell adhesion to activate oxidases. The study found that blocking C1qRp of neutrophils with C1qRp MAb could not inhibit the generation of respiratory bursts, suggesting that poly-C1q binds to another receptor of neutrophils and macrophages and causes respiratory bursts. The region of C1q causing respiratory burst is located in the CLR of C1q, which is different from the site where C1q, MLB, SP-L and C1qRp bind.
-
CRT/ cC1qR/ Collectin Receptor
Calreticulin (CRT) is a single-chain acidic calcium-binding protein that is located in the endoplasmic reticulum of most nucleated cells and participates in the formation of glycoproteins with calnexin. Its ligands are CLR of C1q, MBL, SP-A and conglutinin. Because it binds to the CLR of C1q, this protein is named collagen C1q receptor (cC1qR). C1q CLR combined with CRT can promote FcR and CR1-mediated phagocytosis. Because MBL and SP-A are similar in structure to C1q, they also have a CLR capable of binding to CRT, and after binding to CRT, they have the same functions as C1q. Therefore, CRT is also called a collectin receptor. When cells are stimulated by inflammation, heat shock, viral infection, etc., CRT will be secreted outside the cell, which may cause autoimmune diseases. The binding of IgG-like motifs of CRT to C1q can inhibit the binding of C1q to IC, thereby inhibiting complement activation, indicating that it plays an important role in C1q-mediated complement activation and IC clearance.
-
CR1(CD35)
Human CR1 exists on the surface of white blood cells and red blood cells. Its main function is to recognize ICs conditioned by C3b and C4b, and to clear ICs in the liver and spleen. It was found that CR1 has binding sites for Clq, C3b, and C4b, and that it binds to CLR of C1q. Although Clq, C3b, and C4b all have the effect of clearing IC, C3b and C4b are still the leading ones.
Creative Biolabs Provides High-quality C1q Related Products
Creative Biolabs offers a number of complement system-realted services. If additional help is needed, please directly contact us and consult our technical supports for more details.
Reference
-
Ghebrehiwet B.; et al. Monocyte Expressed Macromolecular C1 and C1q Receptors as Molecular Sensors of Danger: Implications in SLE. Front Immunol. 2014 Jun 26;5:278.
Related Product
For Research Use Only.
Related Sections:

