Enzymatic Conjugation
Over the years, Creative Biolabs has closely monitored advancements in the field of bioconjugation. We are pleased to share cutting-edge insights and technologies related to bioconjugation with our clients.
Enzymes have emerged as a versatile tool in the development of Antibody-Drug Conjugates (ADCs) and protein labeling. These biocatalysts offer high specificity, exceptional efficiency, and mild reaction conditions, making them invaluable for targeted coupling of natural or genetically engineered antibodies and small molecule drugs. These enzymes produce a precisely controlled drug-antibody ratio (DAR) by altering the antibody in a site- or amino acid sequence-specific way.
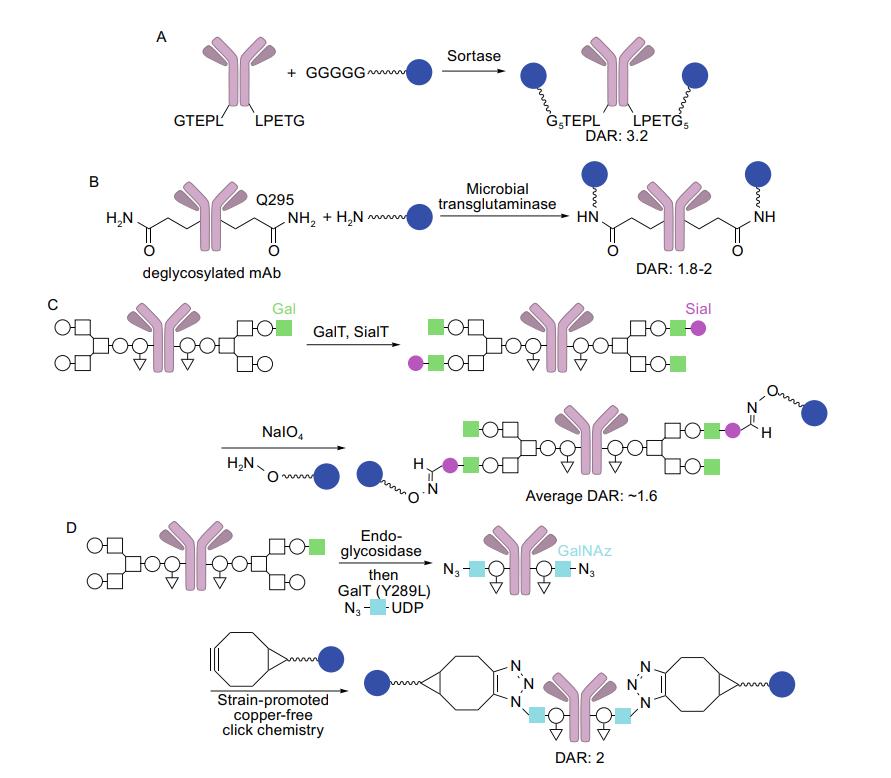 Fig.1 Site-specific enzymatic conjugation.1, 2
Fig.1 Site-specific enzymatic conjugation.1, 2
 Peptidase-Mediated Conjugation
Peptidase-Mediated Conjugation
- Subtiligase
Subtiligase is a variant of subtilisin BPN'. This enzyme, a serine protease, catalyzes the specific modification of various peptides and proteins in aqueous solution with high efficiency by selectively attaching an acyl-donor peptide ester to the N-terminal amine group of an acceptor peptide.
 Transferase-Mediated Conjugation
Transferase-Mediated Conjugation
- Microbial Transglutaminase (MTG)
MTG can catalyze the formation of stable isopeptide bonds between glutamine and lysine, enabling the effective crosslinking of target proteins. Q-tag and K-tag are potent substrates for MTG, which can be strategically incorporated into the N-terminus, C-terminus, or the internal loop of protein.
- N-Myristoytransferase (NMT)
NMT is a robust tool for site-specific protein modification at the N-terminus, which facilitates the covalent crosslinking of myristoyl coenzyme A to the N-terminal glycine residue of specific protein sequences. This co-translational or post-translational modification is recognized by a short peptide motif typically comprising 6-10 amino acids.
- Farnesyltransferase (FTase)
FTase recognizes the CaaX motif, a specific amino acid sequence located at the C-terminus of target proteins, and catalyzes the conjugation of isoprenoid analogs to the cysteine residue within this motif. The unique properties of FTase make it particularly well-suited for modifying proteins where the C-terminus is situated far from the primary site of action, as well as for applications requiring a high degree of product homogeneity.
- Phosphopantetheinyl Transferase (PPTase)
PPTase plays a crucial role in the post-translational modification of acyl carrier protein (ACP) and peptidyl carrier protein (PCP), which are essential domains of several synthetases. This enzyme catalyzes the transfer of the phosphopantetheine group from coenzyme A to conserved serine residues in ACP and PCP, enabling it to serve as a versatile platform for the site-specific modification of target proteins.
 Ligase-Mediated Conjugation
Ligase-Mediated Conjugation
- Tubulin Tyrosine Ligase (TTL)
TTL is a cytoplasmic enzyme that catalyzes the amidation of functionalized tyrosine analogs to the C-terminal carboxyl group of those proteins containing the Tub-tag (a short peptidic recognition sequence) via an ATP-dependent reaction, enabling one-step fluorescent labeling of functional biomolecules.
- Lipoic Acid Ligase (LplA)
LplA can specifically identify the amino acid sequence (GFEIDKVWYDLDA) within the LplA receptor peptide (LAP), which catalyzes the conjugation of lipoic acid analogs to lysine residues in the LAP. This LplA-mediated enzyme-protein modifying process has found numerous applications, including antibody fluorescent labeling, cell surface modification, and protein immobilization.
- Biotin Ligase
Biotin ligase is a crucial enzyme that catalyzes biotinylation reactions, facilitating the conjugation of biotin to specific proteins. The Escherichia coli biotin ligase (BirA) is a widely utilized type of biotin ligase, which can couple biotin derivatives to target proteins through the lysine of the receptor peptide (GLNDIFEAQKIEWHE).
References
- Tsuchikama, Kyoji, and Zhiqiang An. "Antibody-drug conjugates: recent advances in conjugation and linker chemistries." Protein & cell 9.1 (2018): 33-46.
- Distributed under Open Access license CC BY 4.0, without modification.
