Site-Specific Protein Conjugation Targeting Tyrosine
Tyrosine carries a phenolic hydroxyl group and is an aromatic polar alpha-amino acid. Tyrosine is a great target for site-specific alterations since it has amphiphilic characteristics and is comparatively less exposed on the surface of proteins than lysine. Additionally, tyrosine residues exist independently on the surfaces of natural proteins, often in a reactive state, unlike cysteine residues. Therefore, the development of novel modification techniques for tyrosine can complement existing conjugation strategies involving cysteine and lysine, expanding the toolkit for protein functionalization.
Chemical Methods for Tyrosine Conjugation
 Mannich Reaction
Mannich Reaction
The Mannich reaction is a three-component reaction involving the condensation of amines and aldehydes in the presence of acid, leading to the formation of an imine intermediate that acts as an electrophile. Subsequently, enol forms of carbonyl compounds can undergo nucleophilic attack on this intermediate, resulting in C-C bond formation. This method exhibits low selectivity towards tyrosine, resulting in potential nucleophilic attacks by tryptophan and cysteine residues on the imine intermediate. The use of cyclic imines can enhance the selectivity of the reaction. However, they still react with thiols. Thus, this approach allows for site-specific conjugation of tyrosine in the absence of cysteine.
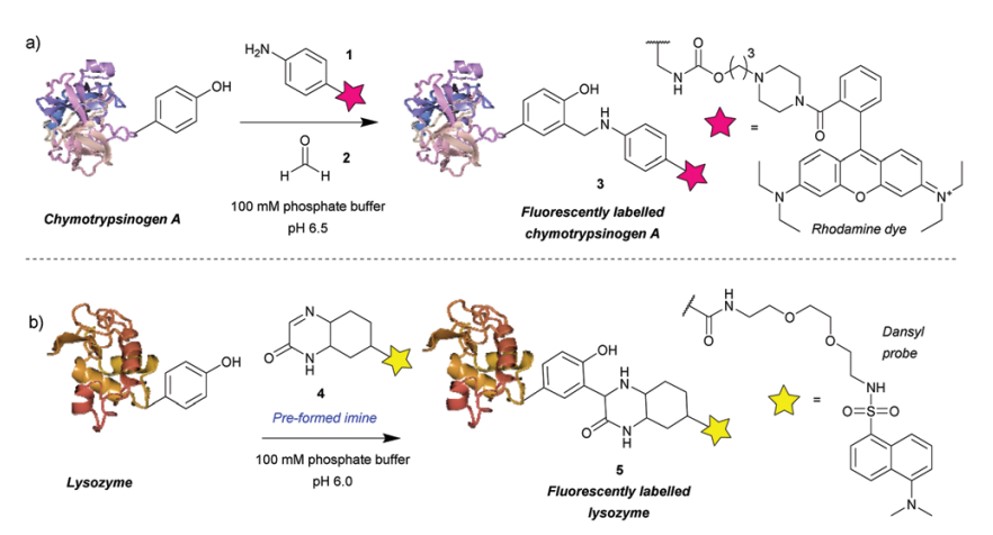 Fig.1 Tyrosine bioconjugation via Mannich reaction.1,2
Fig.1 Tyrosine bioconjugation via Mannich reaction.1,2
 Diazodicarboxyamides
Diazodicarboxyamides
Diazodicarboxyamides, such as 4-phenyl-3H-1,2,4-triazoline-3,5(4H)-dione (PTAD), represent one of the most widely used methods for tyrosine modification. Compared to other reagents, PTAD offers a faster reaction rate and shorter reaction times, demonstrating excellent selectivity for tyrosine. The resulting bioconjugates exhibit remarkable stability, maintaining integrity for up to a week under extreme conditions such as human plasma, 10% sodium hydroxide/hydrochloric acid, or 120°C high temperature. However, PTAD is unstable in water and easily breaks down into isocyanates, which might cause side reactions at protein N-terminal amines or lysine residues. The incorporation of tris(hydroxymethyl)aminomethane (Tris) buffer effectively removes the isocyanates, thereby reducing unwanted side reactions. Furthermore, this method is orthogonal to conjugations involving lysine and cysteine, enabling dual or even triple modifications based on these three amino acids.
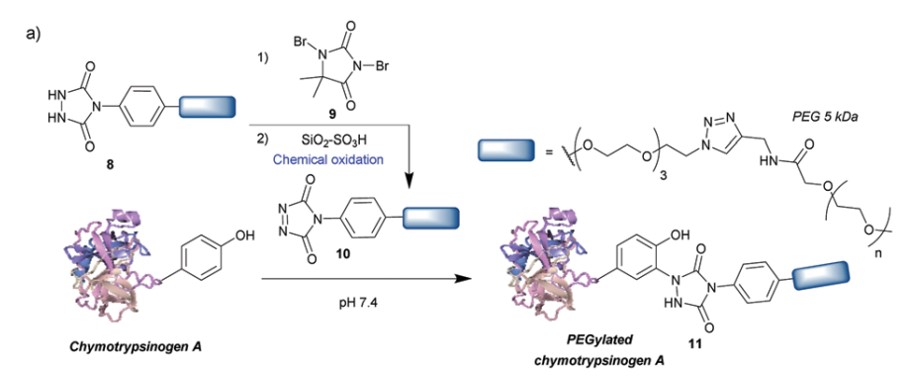 Fig.2 Tyrosine modification strategies based on PTAD.1,3
Fig.2 Tyrosine modification strategies based on PTAD.1,3
 Sulfur Fluoride/Triazole Exchange (SuFEx/SuTEx) Reactions
Sulfur Fluoride/Triazole Exchange (SuFEx/SuTEx) Reactions
Hexavalent sulfur fluorides exhibit high chemical stability and generally remain stable under various chemical conditions, including oxidation, reduction, and hydrolysis. However, in the presence of protonated hydrogen (H+) or silicon-containing compounds (R3Si+), the S-F bond becomes activated, allowing for substitution reactions to occur. SuFEx represents a new generation of click chemistry, functioning under metal-free conditions and demonstrating higher reactivity and specificity towards tyrosine compared to lysine. The substitution of the fluoride leaving group with a triazole group (SuTEx) can further enhance selectivity for tyrosine compared to the S-F bond.
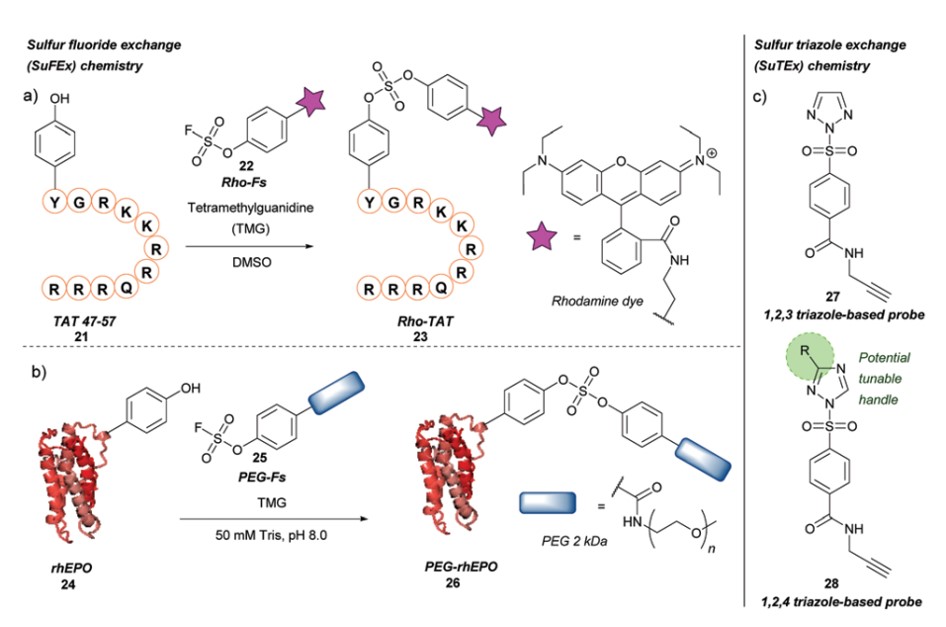 Fig.3 Tyrosine modification based on sulfur fluoride/triazole exchange.1,2
Fig.3 Tyrosine modification based on sulfur fluoride/triazole exchange.1,2
 O-Glycosylation Modification
O-Glycosylation Modification
Fluoroglycosyl donors can be employed for the O-glycosylation modification of tyrosine-containing peptides. These donors facilitate the O-glycosylation of phenolic compounds under aqueous conditions without the need for protecting groups. The inclusion of Ca(OH)2 in the reaction can significantly enhance the reaction rate. However, it is important to note that free cysteine can react with α-D-fluoroglycosyl donors. Therefore, the peptides to be conjugated must not contain free cysteine side chains to prevent the formation of mixed O- and S-glycosylation products.
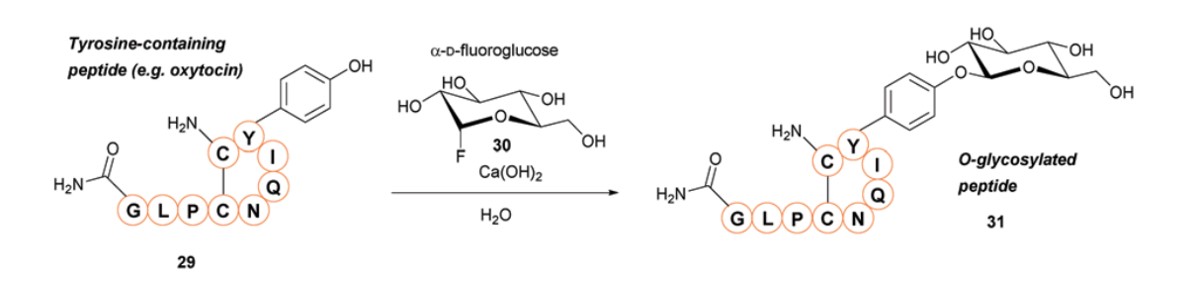 Fig.4 O-glycosylation of tyrosine residues.1,2
Fig.4 O-glycosylation of tyrosine residues.1,2
Enzyme-mediated Methods for Tyrosine Conjugation
In addition to direct chemical conjugation, enzymatic transformation methods have been utilized for the modification of tyrosine. For instance, tyrosinase can oxidize tyrosine to form catechol, which can subsequently undergo O-alkylation or react with functionalized boronic acids to produce conjugates. These catechols can be further oxidized by tyrosinase to yield o-quinones, which may participate in strain-promoted azide-alkyne cycloaddition (SPAAC) with benzocyclooctyne (BCN), or react with sulfur or nitrogen nucleophiles. Furthermore, tubulin tyrosine ligase (TTL) can specifically recognize the "Tub tag" at the C-terminus of tubulin, allowing for the attachment of non-natural tyrosine derivatives for subsequent modifications. Additionally, deoxyribozymes can facilitate the modification of tyrosine by catalyzing its nucleophilic attack on triphosphates, enabling conjugation between RNA and peptides, or introducing other functional groups (e.g., azide groups) for further specific modifications.
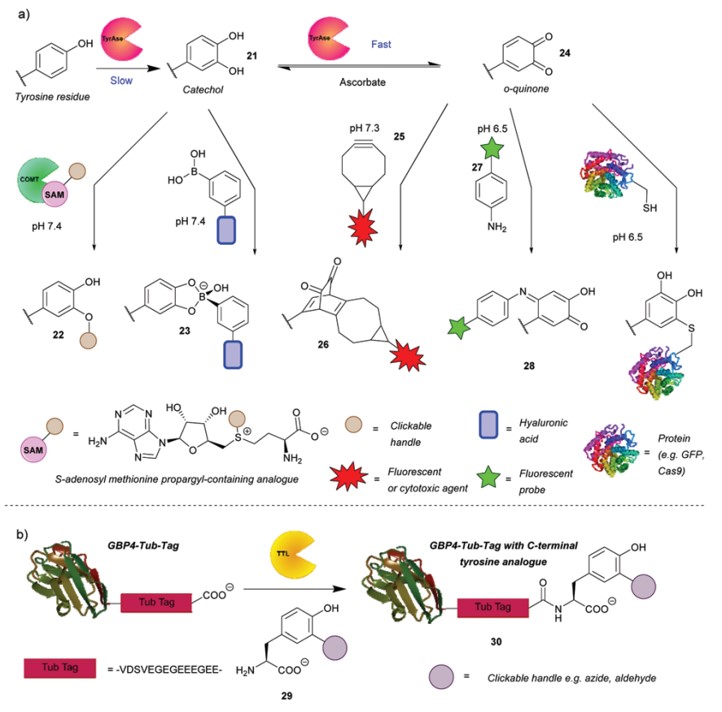 Fig.5 Enzyme-mediated strategies for tyrosine bioconjugation.1,2
Fig.5 Enzyme-mediated strategies for tyrosine bioconjugation.1,2
References
- Szijj, Peter A., et al. "Tyrosine bioconjugation–an emergent alternative." Organic & Biomolecular Chemistry 18.44 (2020): 9018-9028.
- Distributed under Open Access license CC BY 3.0, without modification.
- Distributed under Open Access license CC BY 3.0. The image was modified by extracting and using only Part A of the original image.
