Bioorthogonal Chemistry
Introduction of Bioorthogonal Chemistry
Bioorthogonal reactions are a class of chemical reactions that can occur within biological systems, particularly in living organisms, without interfering with surrounding biochemical processes. There are two primary steps in these bioorthogonal processes: Using methods like metabolic labeling, biomolecules are first modified to contain a bioorthogonal handle, such as an azide group. The external probe is then introduced with a functional group, like an alkyne, which links with the bioorthogonal handle quickly and selectively to produce conjugate. The concept of bioorthogonal chemistry was formally introduced by Carolyn Bertozzi in 2003, highlighting the simplicity, efficiency, and selectivity of these reactions. Over the past two decades, bioorthogonal chemistry has become one of the most important tools in fields such as chemical synthesis, biological labeling, and materials preparation.
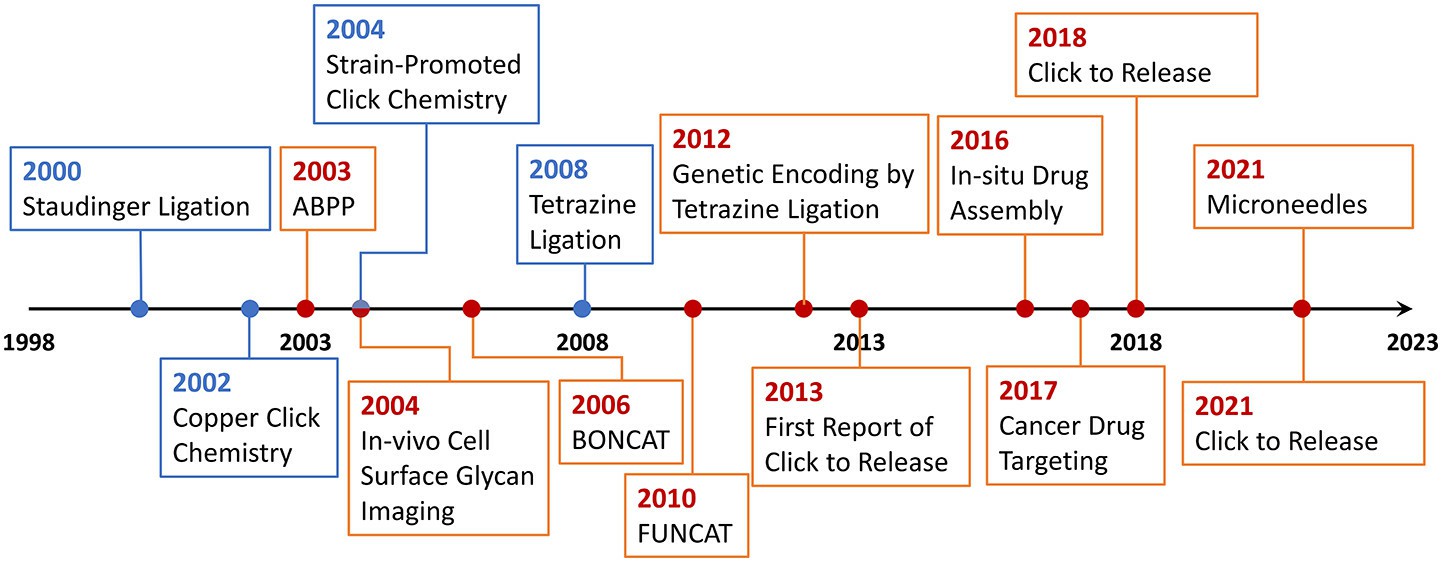 Fig.1 The development timeline of bioorthogonal chemistry.1
Fig.1 The development timeline of bioorthogonal chemistry.1
Advantageous Properties of Bioorthogonal Chemistry
- High selectivity: The design of bioorthogonal reactions enables high specificity and yield, effectively avoiding interference from endogenous nucleophiles, electrophiles, oxidants, or reductants present in aqueous or complex biological environments.
- Excellent reactivity: These reactions demonstrate remarkable reactivity, enabling rapid progress even at low concentrations and leading to the formation of stable end products.
- Gentle reaction conditions: Bioorthogonal reactions can occur under mild conditions, maintaining physiological temperature and pH levels.
- Excellent biocompatibility: Many bioorthogonal reactions do not require heavy metal catalysts, ensuring excellent biocompatibility, which makes them well-suited for applications in live cells or biological systems.
Conjugation Methods Based on Bioorthogonal Chemistry
Click Reaction
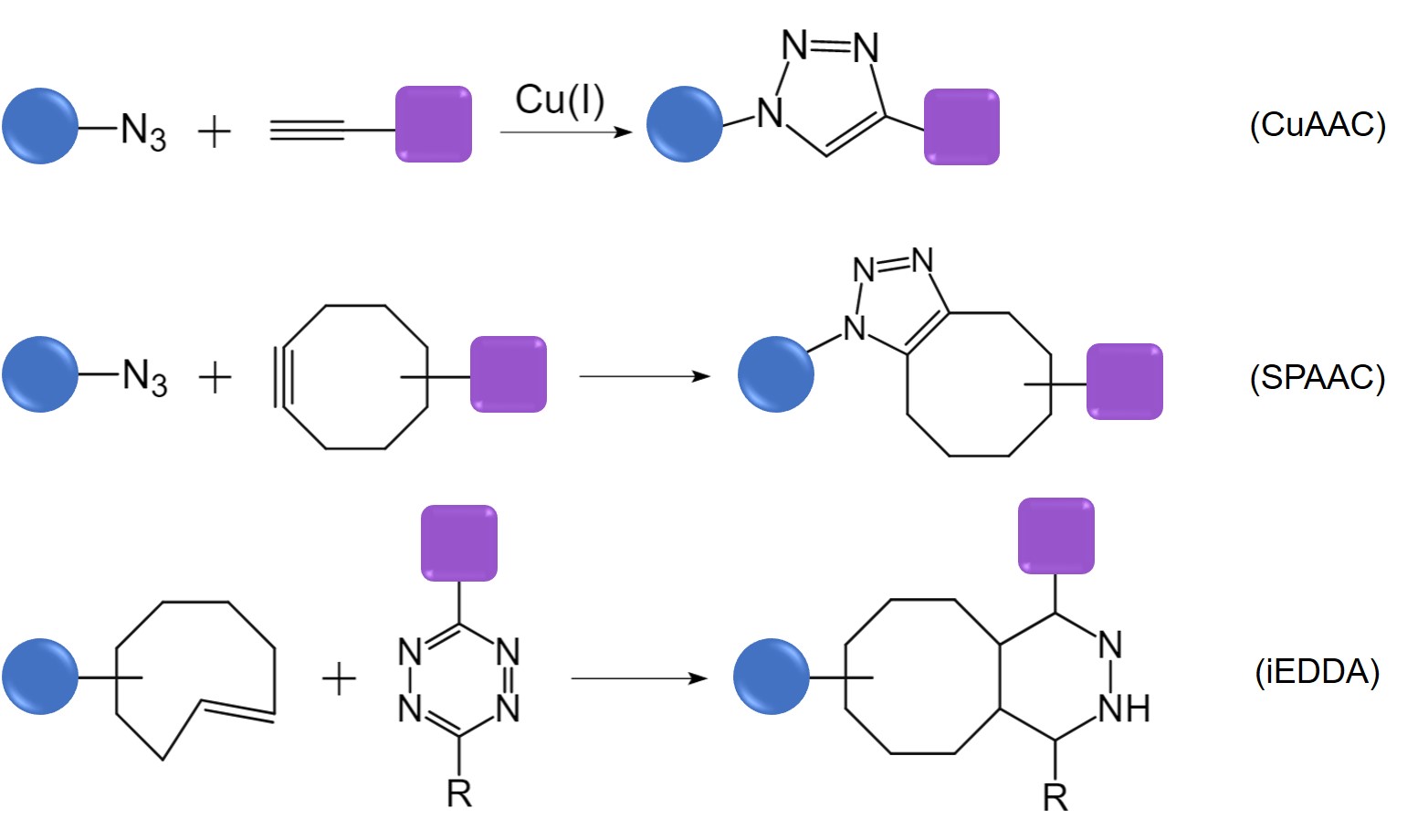
The metal-catalyzed cycloaddition reaction (CuAAC) is one of the earliest and most extensively studied bioorthogonal reactions. However, due to the cytotoxicity associated with copper ions (Cu+), researchers have modified alkyne substrates to develop the copper-free variant known as SPAAC. Additionally, the iEDDA-based tetrazine ligation represents another rapid bioorthogonal reaction, characterized by its rapid kinetics, making it particularly suitable for applications in live cells.
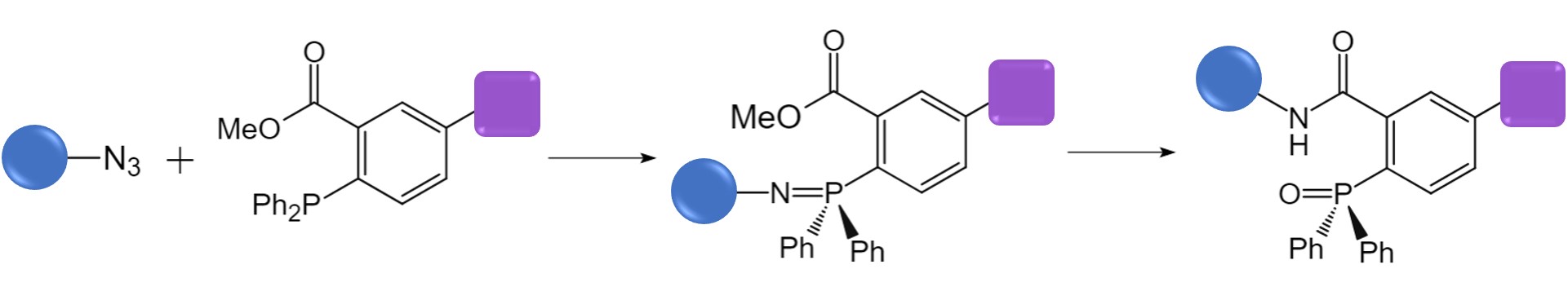
Staudinger Ligation
The Stinger reaction is among the earliest bio-orthogonal reactions developed. In this process, azides react with phosphines to yield iminophosphorane intermediates. However, these intermediates are unstable and hydrolyze to form amines and phosphine oxides as final products. Bertozzi enhanced this classic reaction by employing organic azides equipped with electrophilic traps, like methyl esters. This modification allows the iminophosphorane to react with the neighboring trap, facilitating the formation of the desired conjugate through an amide bond.
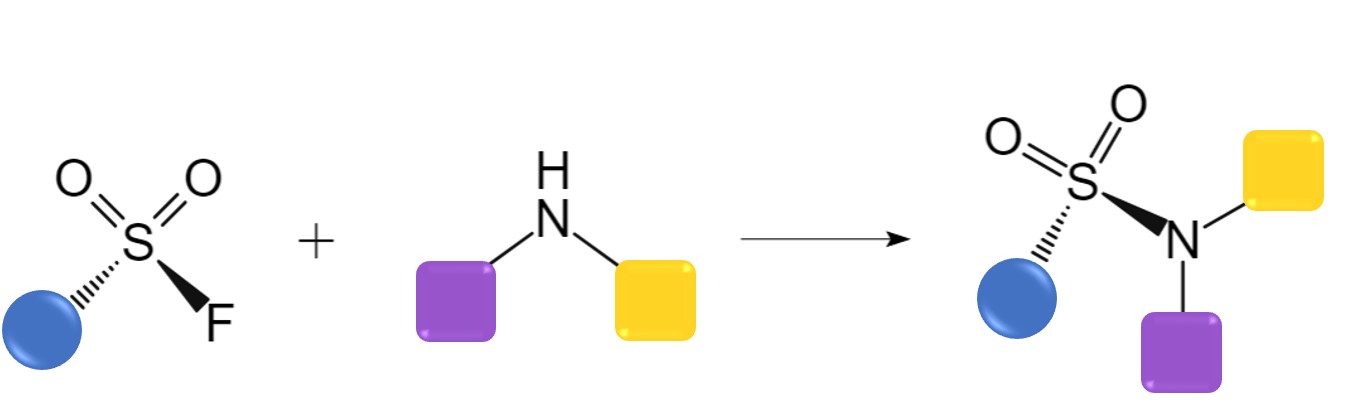
Sulfur(VI)-Fluoride Exchange (SuFEx)
In SuFEx reaction, the S-F bond exhibits outstanding stability compared to the more common S-Cl bond, enabling the reaction to maintain orthogonality in complex environments. Under appropriate conditions, such as activation by protons or silicon, the S-F bond demonstrates exceptional reactivity towards nucleophiles containing O or N, facilitating the formation of S(VI)-O or S(VI)-N bonds. The accessibility of starting materials, simplicity of operations, and scalability further contribute to the widespread application of SuFEx reaction in various fields.
Creative Biolabs is pleased to share cutting-edge advancements in bioorthogonal chemistry with our clients. We also offer conjugation services based on this innovative approach—Click Chemistry Based Conjugation. If you are interested in specific conjugation services, their application cases, or how to incorporate these techniques into your projects, please contact us for further information whenever you need it.
References
- Bird, Robert E., et al. "Bioorthogonal chemistry and its applications." Bioconjugate Chemistry 32.12 (2021): 2457-2479. Distributed under Open Access license CC BY 4.0, without modification.
Related Products
