What is Vitronectin Vitronectin Function Vitronectin Test Vitronectin in Disease Vitronectin Therapeutics
Vitronectin (VTN), a multifunctional glycoprotein abundantly present in plasma and the extracellular matrix, plays a pivotal role in modulating the complement system. As an essential regulator, VTN helps prevent excessive complement activation that could lead to tissue damage, while simultaneously contributing to cellular adhesion, migration, and tissue remodeling. This dual functional capacity makes vitronectin a molecule of considerable interest in immunology, inflammation, cancer, and biomaterials research.
What is Vitronectin?
Vitronectin, also known as S-protein, is a 75-kDa glycoprotein primarily synthesized by the liver and secreted into the bloodstream. It circulates in a single-chain form and also exists in multimeric configurations within tissue matrices. Vitronectin belongs to the hemopexin superfamily and contains multiple binding domains that interact with various ligands, including integrins, plasminogen activator inhibitor-1 (PAI-1), and complement system proteins.
Vitronectin (VTN) is synthesized as a precursor polypeptide consisting of 478 amino acids (aa). The precursor protein sequence consists of a 19 aa signal peptide and 459 aa mature protein.
Table 1 The physicochemical properties of the mature protein
|
pI
|
4.75-5.25
|
|
Mr (K) Predicted
|
52.5
|
|
Mr (K) Observed
|
~75
|
|
N-linked glycosylation sites
|
3 (86, 169, 242)
|
|
Phosphorylation sites
|
3 (Thr-50, Thr-57, Ser-263)
|
|
Sulphation sites
|
2 (Tyr-56, Tyr-59)
|
The first 43 aa on the VTN molecule is identical to the somatomedin-B (SMB) consensus sequence. This is followed by a triplet residue Arg-Gly-Asp (RGD) (aa residues 45-47) that functions as an anchoring site for cell integrin receptors. Downstream of the RGD sequence is a stretch of highly acidic amino acids (residues 53-64) that has binding affinity for the thrombin-antithrombin III complex (TAT) and collagen. VTN has three heparin-binding sites, namely HBD-1, HBD-2 and HBD-3 that are located at residues 82-137, 175-219, and 346-361, respectively. VTN harbours four hemopexin repeats (Hpx; heme-binding protein in plasma) distributed along the central region (Hpx-1, Hpx-2, and Hpx-3 at residues 142-285) and C-terminal edge (Hpx-4 at residues 406-453). As a complement regulator, more than half of the VTN molecule (residues 51-310) accommodates interaction sites for complement proteins C5b-C7 and C9.
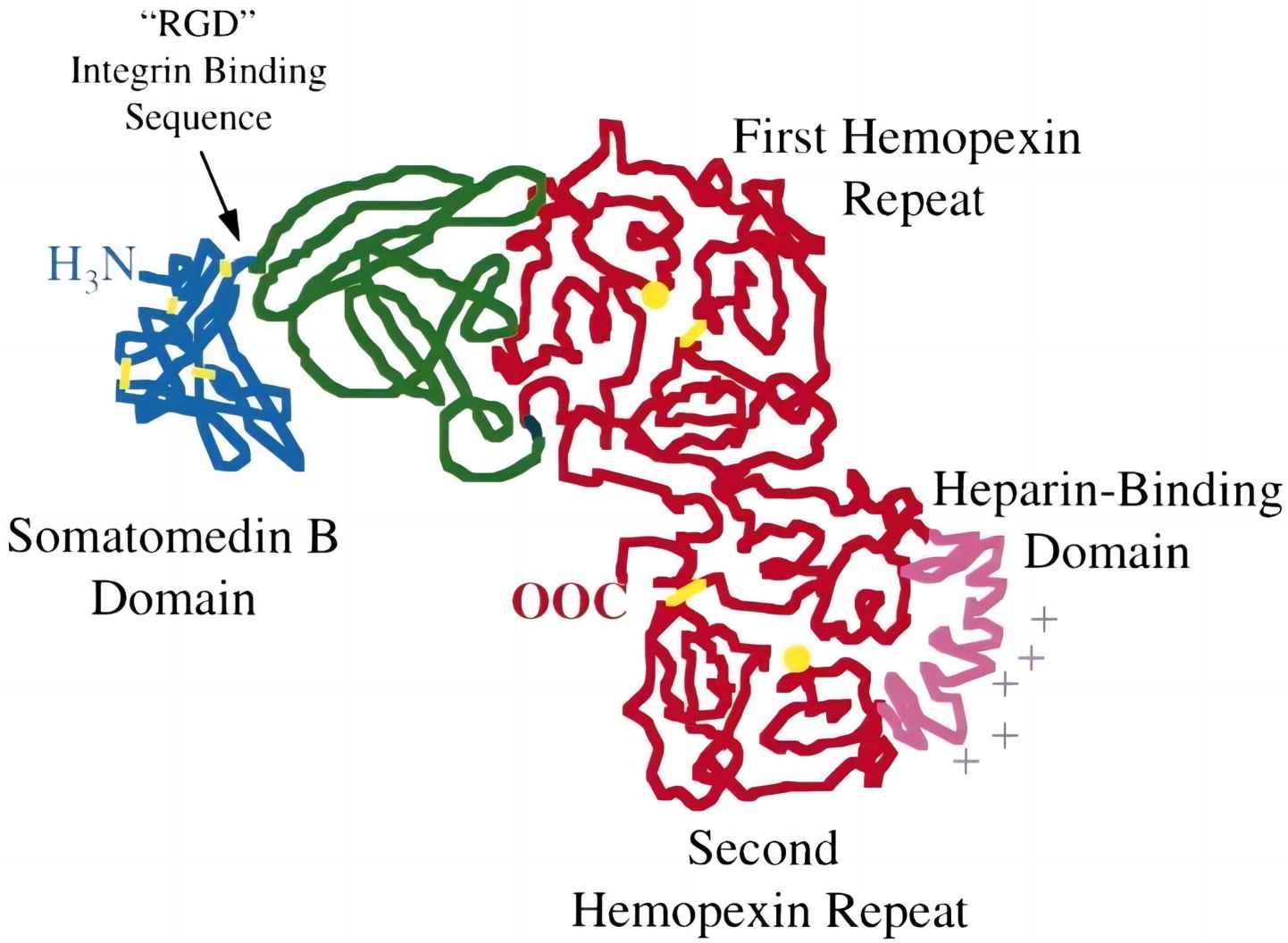 Fig. 1 Domain arrangement of vitronectin.1, 2
Fig. 1 Domain arrangement of vitronectin.1, 2
VTN circulates in two forms in human blood: (1) a 75 kDa-full length single-chain fragment and (2) a disulfide-linked dimer consisting of a two-chain fragment (65 and 10 kDa). The two-chain fragment is a product of the 75-kDa mature protein cleaved by an unknown protease between Arg-379 and Ala-380. The proteolytic products are linked by a single disulfide bridge at residues Cys-274 and Cys-453 of N-terminal of 65 kDa and C-terminal 10 kDa fragments, respectively. In addition, VTN has a high degree of conformational flexible structure. In circulating blood, VTN exists as a heterogeneous mixture consisting of different forms that serve different biological roles, either as monomeric or multimeric VTN.
Biological Functions of Vitronectin
Key biological functions of VTN, mainly in its activated form, include (1) regulation of the innate immune system, (2) maintenance of vascular haemostasis (thrombosis and fibrinolysis), and, finally, (3) promotion of cell adhesion and migration in tissue repair and regeneration. To protect host cells from innocent bystander cell lysis (self-killing), VTN functions as a regulator at the terminal lytic step of the (1) complement pathway and (2) perforin-mediated cell lysis. In the terminal complement pathway, VTN interacts with the premembrane attack complex (pre-MAC) of C5b-7 that in turn occupies the metastable membrane binding site of the complex. This eventually hinders the insertion of the C5b-7 into the cell membrane and thus precludes the downstream completion with C9 to form the MAC lytic pore (C5b-9). Another regulatory mechanism is via interaction of free (nonmembrane inserted) C5b-7, C5b-8 and C5b-9 complex with VTN into water-soluble complexes (sC5b-7, etc.) that are haemolytically nonactive.
VTN contributes to innate immune modulation by inhibiting the terminal complement pathway:
VTN's role extends well beyond immune regulation. Key physiological functions include:
-
Cell adhesion and migration: via RGD motif binding to integrins.
-
Wound healing: promoting epithelial and endothelial cell proliferation.
-
Angiogenesis: facilitating vascular endothelial growth.
-
Regulation of fibrinolysis: binding and stabilizing PAI-1.
Vitronectin Functional Test
VTN is mainly synthesized in the liver as a single-chain 75 kDa polypeptide precursor and is released to the circulation and interstitial space or ECM through receptor-mediated trans-, endo- or exocytosis. In addition to the liver as the major organ of VTN biosynthesis, appreciable amounts of VTN deposits or/and its mRNA are also detected in various normal tissues and organs. VTN is present in normal human plasma at a concentration between 200 and 400 μg/mL (constitutes 0.2%-0.5% of total plasma proteins), mainly as a monomeric form and is predominantly derived from the liver. VTN deposited within platelet (mainly as multimeric aggregates) is the second main circulating pool of VTN and accounts for 0.8%-1% of the total circulating VTN. Tissues experiencing stress or trauma had upregulated levels of VTN mRNA as a proinflammatory response with increased VTN accumulated in the extravascular space of the traumatized tissues.
Understanding the functional role of VTN in complement regulation, cell adhesion, and extracellular matrix (ECM) interactions is essential for both basic and translational research. At Creative Biolabs, we provide comprehensive VTN functional testing services to evaluate its biological activities under physiologically relevant conditions.
Table 2 Key assay formats for VTN functional test.
|
Test
|
Purpose
|
Output
|
|
C5b-7 Binding Inhibition Assay
|
Assess VTN's ability to block MAC formation
|
Quantitative inhibition curves
|
|
Integrin Binding Assay (e.g., αvβ3, αvβ5)
|
Determine cell adhesion via RGD-mediated binding
|
IC50/EC50 values
|
|
SPR or BLI
|
Analyze molecular interactions with complement components
|
Affinity constants (KD)
|
Vitronectin in Health and Disease
VTN plays a central role in maintaining physiological balance across a variety of systems, including immunity, coagulation, wound healing, and extracellular matrix remodeling. However, dysregulation or abnormal accumulation of VTN has been implicated in numerous disease states. Understanding these associations not only provides insights into pathogenesis but also opens new avenues for diagnostic and therapeutic intervention.
Molecular Mechanisms Driving Pathology
-
Complement dysregulation: Excess vitronectin may stabilize sC5b-9, preventing proper clearance and leading to sustained inflammation.
-
Cellular hyper-adhesion: In tumors or fibrotic tissues, overexpressed VTN amplifies integrin signaling and ECM stiffness.
Disease Associations
Table 3 Disease associations.
|
Disease
|
Vitronectin Role
|
|
Age-Related Macular Degeneration (AMD)
|
VTN accumulates in drusen and binds complement components (C5b-9), contributing to chronic inflammation and retinal degeneration
|
|
Cancer (e.g., breast, colon, glioblastoma)
|
Promotes tumor cell adhesion, migration, angiogenesis, and immune evasion by binding integrins and inhibiting MAC
|
|
Sepsis and Systemic Inflammation
|
Elevated plasma VTN levels correlate with complement inhibition and endothelial activation
|
|
Fibrosis (e.g., liver, lung)
|
Supports fibroblast adhesion and ECM remodeling via integrin signaling
|
|
Atherosclerosis
|
Localizes in atherosclerotic plaques, supporting foam cell adhesion and smooth muscle proliferation
|
Vitronectin as a Target in Drug Development
Given its multifaceted roles in immunity, angiogenesis, cell adhesion, and complement regulation, VTN has emerged as a promising therapeutic target across multiple disease domains. Its ability to interact with both immune and structural components of the extracellular matrix positions it at the crossroads of inflammation, cancer progression, and tissue remodeling.
Strategies targeting VTN include:
-
Anti-vitronectin antibodies and small-molecule inhibitors
-
VTN-targeted peptides
-
Therapeutic VTN modulation
As a pioneer in complement system research and immunological assay development, Creative Biolabs offers an end-to-end platform to accelerate VTN-targeted drug discovery.
If you want more information, please feel free to contact us.
References
-
Gibson, Angelia D., et al. "Orientation of heparin-binding sites in native vitronectin: analyses of ligand binding to the primary glycosaminoglycan-binding site indicate that putative secondary sites are not functional." Journal of Biological Chemistry 274.10 (1999): 6432-6442. https://doi.org/10.1074/jbc.274.10.6432
-
under Open Access license CC BY 4.0, without modification
For Research Use Only.
Related Sections:

 Fig. 1 Domain arrangement of vitronectin.1, 2
Fig. 1 Domain arrangement of vitronectin.1, 2