Creative Biolabs provides contract services for complement research. The broad base of experience of our employees, as well as our ability to assemble multidisciplinary project teams, has repeatedly proved to be invaluable assets in carrying out new R&D projects. Here we briefly summarize the esterolytic assay Protocol using different substrates.
Esterolytic Assay Protocol
-
ZLNE Substrate
Functional activities of C1r and C1s can be measured using the chromogenic substrate ZLNE.
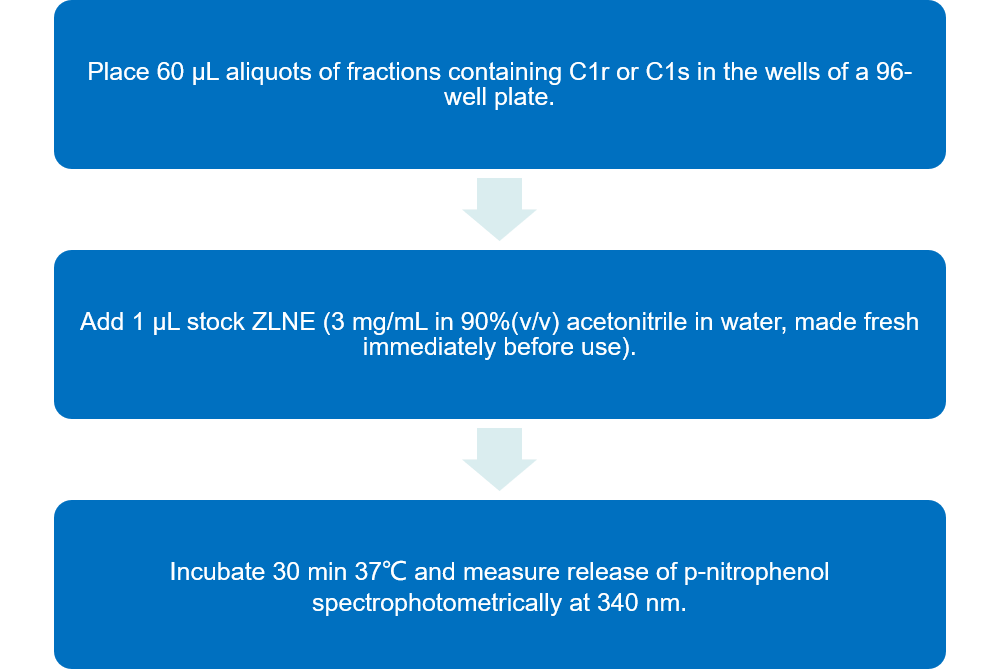
Fig.1 Flow chart of esterolytic activity analysis of C1r and C1s protocol by using substrate ZLNE. (Creative Biolabs)
-
AGLMe Substrate
The following assay uses the AGLMe substrate and is based on indirect measurement of methanol released upon cleavage of the methyl ester.
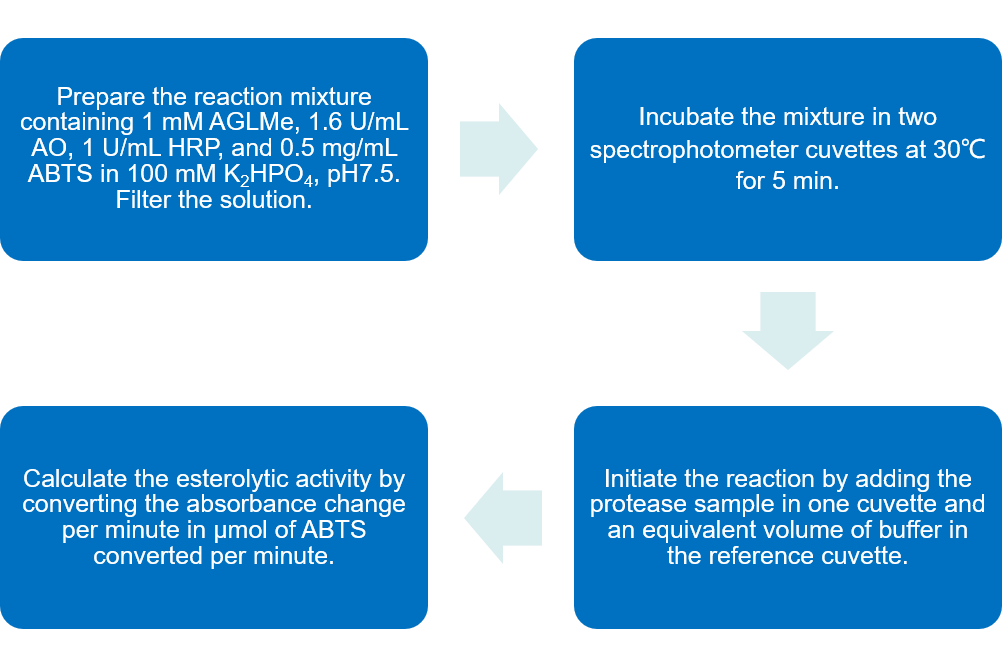
Fig.2 Flow chart of esterolytic activity analysis of C1r and C1s protocol by using substrate AGLMe. (Creative Biolabs)
-
BAEe Substrate
The following assay uses the BAEe substrate and is based on indirect measurement of ethanol released upon cleavage of the ethylester. This assay is less sensitive than the AGLMe assay but is specific for C1s since BAEe is not cleaved by C1r.
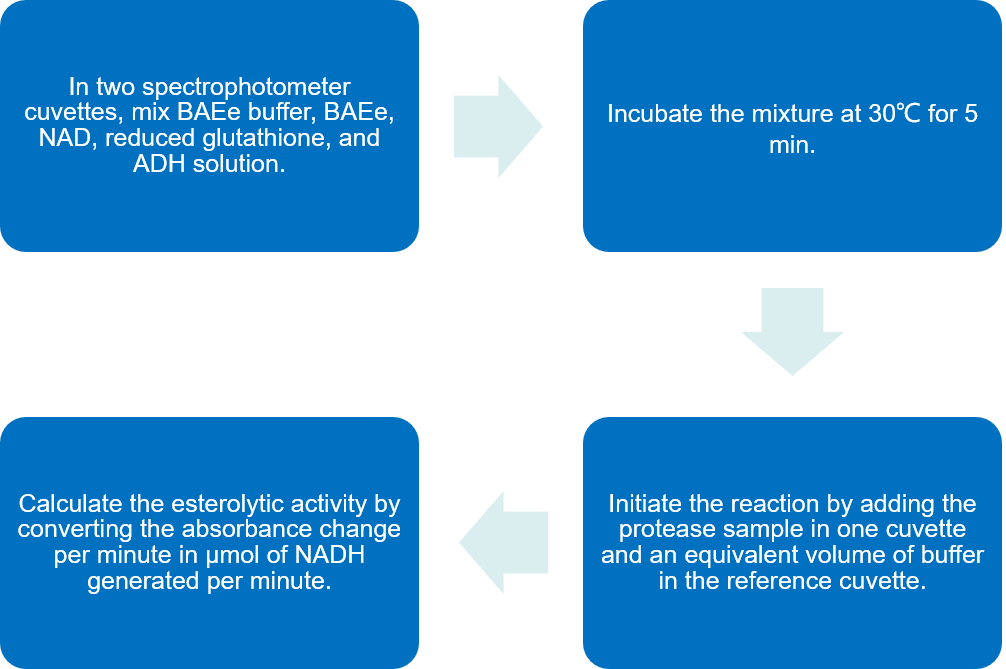
Fig.3 Flow chart of esterolytic activity analysis of C1r and C1s protocol by using substrate BAEe. (Creative Biolabs)
Published Data
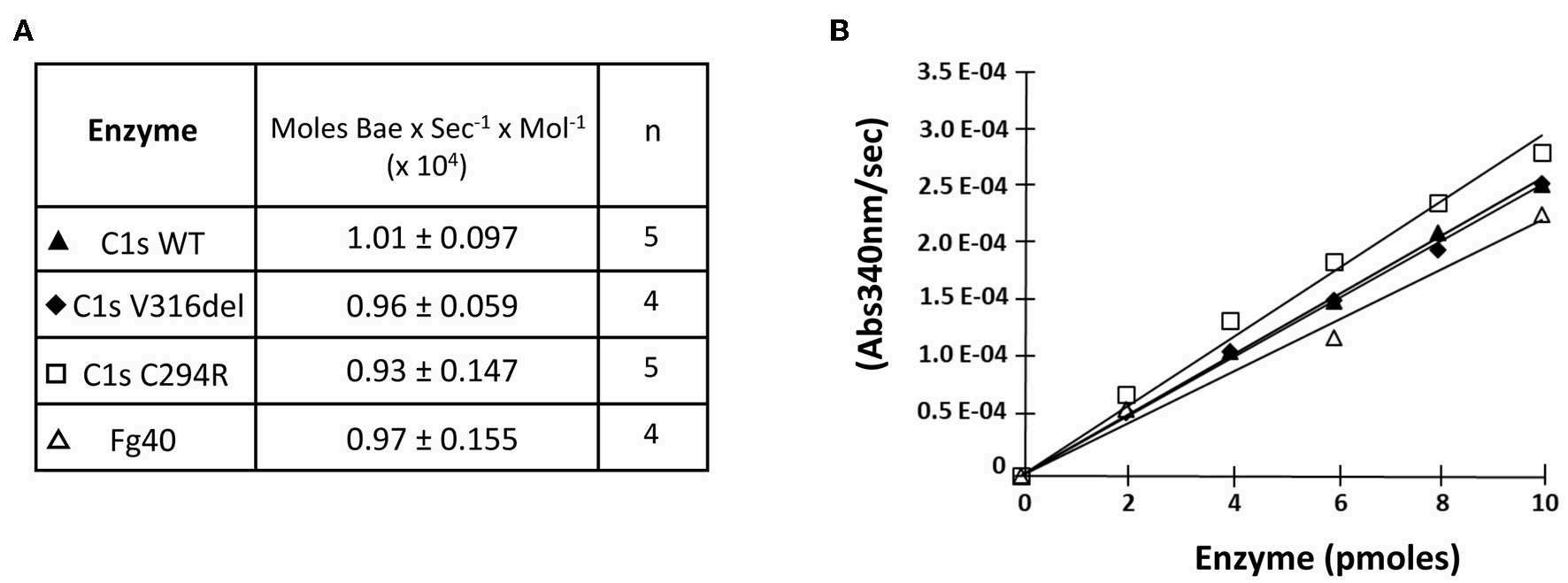 Fig.4 Esterolytic properties of pEDS C1s variants and the Fg40 fragment.1
Fig.4 Esterolytic properties of pEDS C1s variants and the Fg40 fragment.1
Researchers evaluated whether the catalytic function of all pEDS-associated C1s variants was preserved by examining the esterolytic cleavage of BAEe. The variants demonstrated similar substrate hydrolysis efficiency as C1sWT and Fg40, with a specific activity of approximately 1 x 104 moles of substrate hydrolyzed per second per mole of enzyme. These findings emphasize that pEDS variants result in a secreted fragment retaining native esterolytic activity. This discovery paves the way for further molecular studies, potentially exploring additional C1s targets.
Complement-related Services and Products
-
Complement Products
-
Complement Test Services
At Creative Biolabs, we are committed to providing best-in-class testing services and high-quality products for our customers' complement testing needs. We are passionate about providing high-quality service, fast turnaround times, and excellent customer support. If you are interested in our complement services or products, please do not hesitate to contact us for more details.
Please note that our protocols are only for your reference!
Reference
-
Bally, Isabelle, et al. "Two different missense C1S mutations, associated to periodontal Ehlers-Danlos syndrome, lead to identical molecular outcomes." Frontiers in Immunology 10 (2019): 2962. Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
Related Sections:




 Fig.4 Esterolytic properties of pEDS C1s variants and the Fg40 fragment.1
Fig.4 Esterolytic properties of pEDS C1s variants and the Fg40 fragment.1