Creative Biolabs is one of the well-recognized experts who are professional in applying advanced platforms and technologies for a broad range of complement-related objectives. It has been proved that mutation of the active site serine 637 to alanine allows the production of recombinant C1r stabilized in the proenzyme form and then allows to reconstitute of a recombinant C1s–C1r–C1r–C1s tetramers. Here, we describe the recombinant C1s-C1r-C1r-C1s tetramer purification protocol to promote your research.
Recombinant C1s-C1r-C1r-C1s Tetramer Purification Protocol
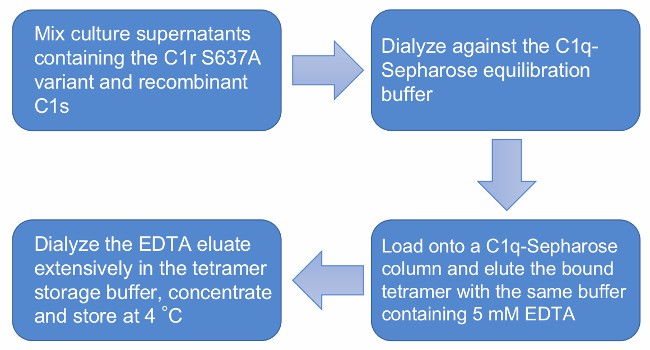
Fig.1 Flow chart of recombinant C1s-C1r-C1r-C1s tetramer purification. (Creative Biolabs)
Published Data
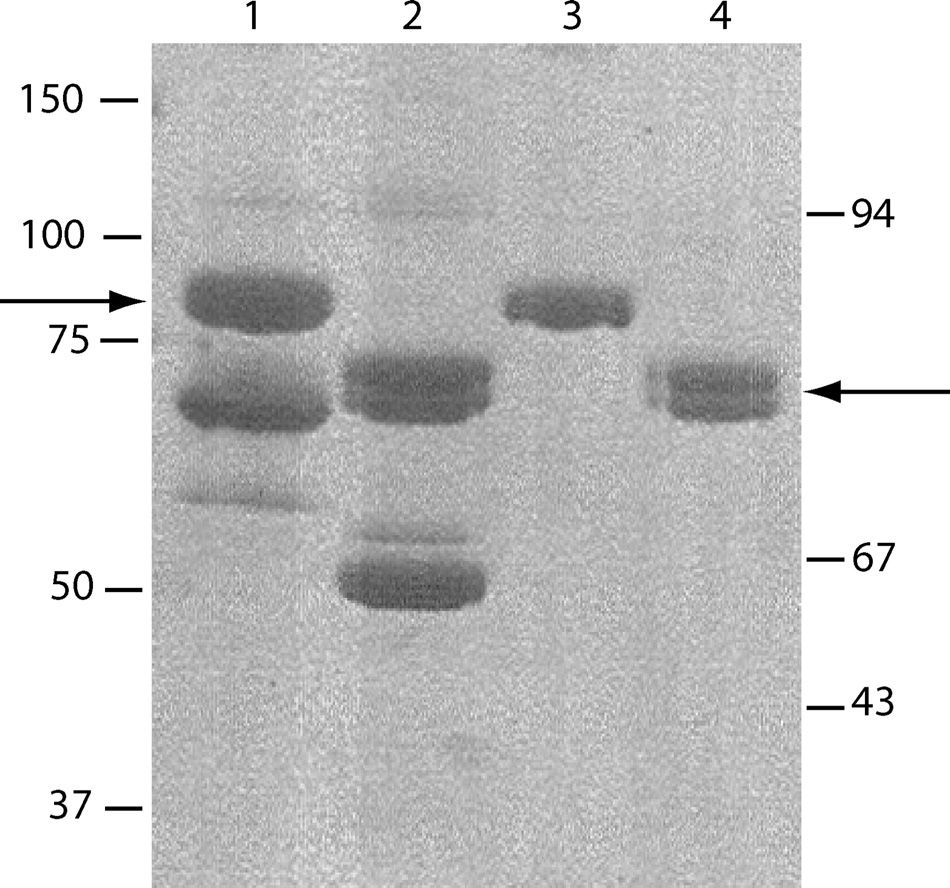 Fig.2 Isolation of the recombinant C1s-C1r-C1r-C1s tetramer.1
Fig.2 Isolation of the recombinant C1s-C1r-C1r-C1s tetramer.1
The SDS-PAGE analysis, followed by Coomassie Blue staining and gel scanning, was employed to quantify the relative amounts of C1r or C1s in each culture supernatant. Based on this analysis, C1r- and C1s-rich supernatants were combined to reach an approximate 1:1 C1r:C1s ratio. The mixture was dialyzed and subsequently loaded onto a column pre-equilibrated with the identical buffer. Elution utilized a linear NaCl gradient. SDS-PAGE analysis identified fractions containing the C1s-C1r-C1r-C1s tetramer, which were pooled and concentrated using ultrafiltration. High-pressure gel filtration on an equilibrated column further purified the tetramer, which was then concentrated to 0.2 mg/ml by ultrafiltration and stored at 4°C.
Equipped with advanced technologies and platforms, Creative Biolabs has won a good reputation among our worldwide customers for successfully accomplishing numerous challenging projects in the field of the complement system. Now we can offer a full suite of products and services to help pharmaceutical and biotech companies accelerate their research and drug development.
To learn more details, you can get access with the following links:
-
Complement Products
-
Complement Test Services
If you are interested in our products and services, please do not hesitate to contact us for more information.
Please note that our protocols are only for your reference!
Reference
-
Bally, Isabelle, et al. "Identification of the C1q-binding sites of human C1r and C1s: a refined three-dimensional model of the C1 complex of complement." Journal of Biological Chemistry 284.29 (2009): 19340-19348. Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
Related Sections:


 Fig.2 Isolation of the recombinant C1s-C1r-C1r-C1s tetramer.1
Fig.2 Isolation of the recombinant C1s-C1r-C1r-C1s tetramer.1