CEA Promoter driven Oncolytic Adenovirus Construction Service
Introduction
Our CEA Promoter-driven Oncolytic Adenovirus Service overcomes conventional cancer therapy limitations like low specificity and side effects via advanced gene-virotherapy. Using tumor-specific promoters and synergistic multi-gene payloads, it accelerates targeted tumor suppression development. Creative Biolabs' end-to-end solutions—from custom vector design to in vivo validation—deliver precise oncolytic agents for solid tumors, minimizing off-target effects. The approach ensures targeted gene delivery for tumor regression and apoptosis, maintaining safety through reduced systemic toxicity, with robust preclinical validation to advance therapeutic candidates.
[Discover How We Can Help - Request a Consultation]
CEA Promoter-driven Oncolytic Adenovirus
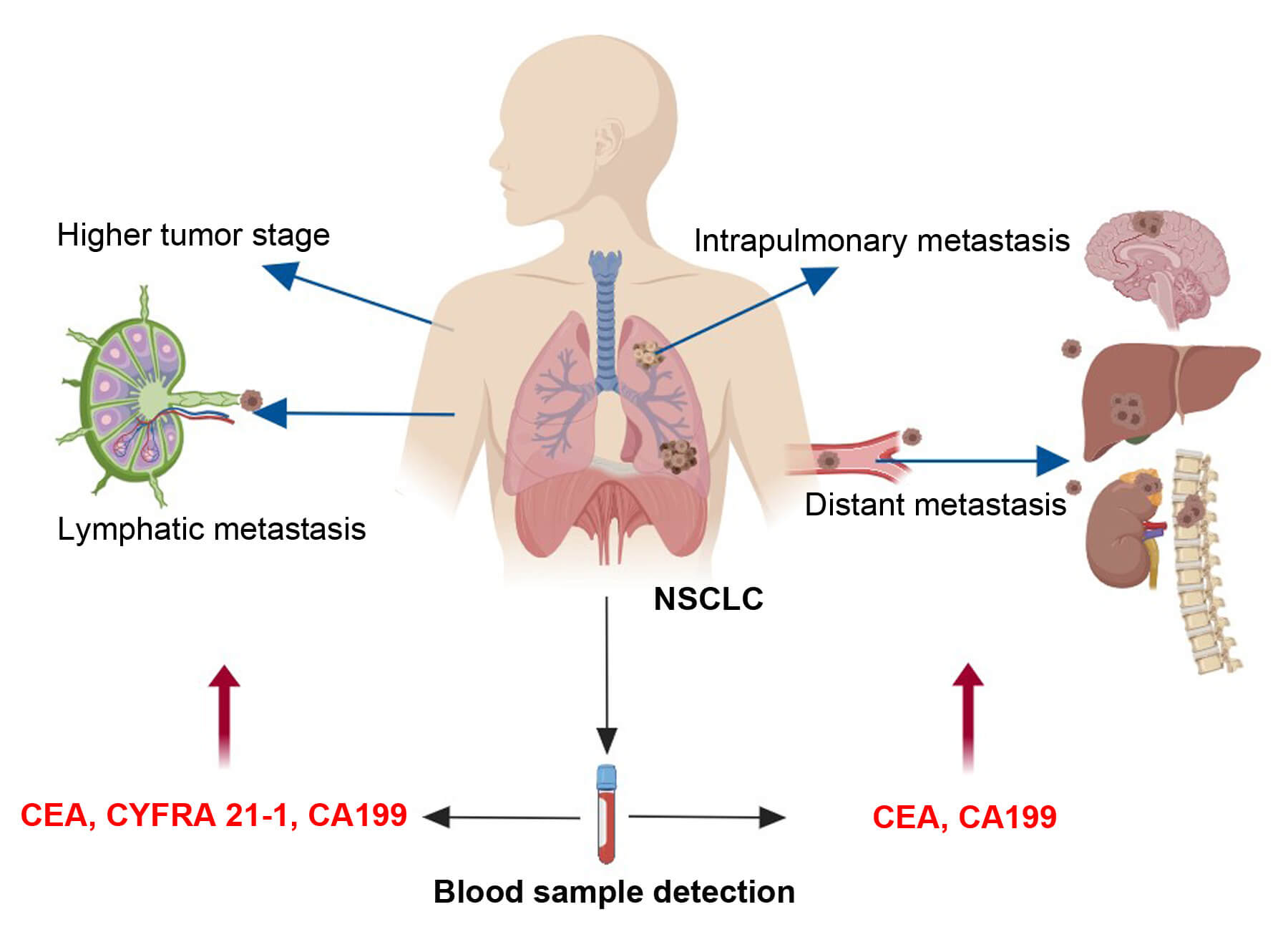 Fig.1 CEA is widely used as a tumor marker to assist in the diagnosis of malignant tumors and monitor the therapeutic effect.1
Fig.1 CEA is widely used as a tumor marker to assist in the diagnosis of malignant tumors and monitor the therapeutic effect.1
Carcinoembryonic Antigen (CEA), a glycoprotein expressed in the fetal intestine and liver, declines post-birth, with normal adult serum levels <5 ng/mL. Its aberrant elevation in cancers like colorectal and lung makes it a tumor marker for diagnosis, treatment monitoring (levels fall with efficacy, rise in recurrence), and targeted therapy. The CEA promoter drives gene therapy vectors (e.g., oncolytic adenoviruses) to express therapeutic genes in CEA-positive tumors, sparing normal tissues.
Principle
CEA Promoter-driven Oncolytic Adenoviruses operate on a sophisticated principle combining selective viral replication with targeted gene expression.
- Oncolytic Virotherapy: At its core, this approach utilizes viruses that are genetically modified to selectively replicate within and lyse cancer cells, while leaving healthy, non-malignant cells unharmed. This inherent tumor selectivity is a cornerstone of oncolytic virotherapy.
- CEA Promoter Control: The CEA promoter is key to tumor specificity. CEA is an oncofetal protein overexpressed in lung, colorectal, and gastric carcinomas. In our engineered adenovirus, the viral replication-critical E1A gene is controlled by the CEA promoter, allowing replication and therapeutic payload expression only in CEA-overexpressing cancer cells.
- Dual Gene Payload (e.g., TRAIL-IETD-MnSOD): To enhance efficacy, CEA promoter-driven oncolytic adenoviruses carry therapeutic genes like TRAIL and MnSOD. TRAIL induces cancer cell apoptosis, while MnSOD boosts its effect by modulating oxidative stress. Linked via an IETD caspase-8 cleavage site, the genes enable co-expression and synergistic antitumor action in the tumor microenvironment.
Advantages
- High Tumor Specificity: CEA promoter restricts viral replication and gene expression to CEA-positive cells, reducing systemic toxicity and off-target effects.
- Potent Antitumor Efficacy: Combines direct oncolytic lysis with synergistic therapeutic genes for multifaceted tumor regression and enhanced apoptosis.
- Enhanced Safety Profile: Tumor-specific targeting minimizes replication in normal cells, yielding favorable preclinical safety and lower adverse event risk.
- Immune Stimulation: Oncolysis releases tumor antigens and danger signals, triggering robust anti-tumor immune responses and overcoming immune evasion.
- Versatility: Adaptable to different solid tumors by switching promoters (for other markers) and capable of carrying multiple therapeutic genes to address cancer hallmarks/resistance.
Workflow
| Required Starting Materials | Vector Design & Gene Cloning |
|---|---|
|
Custom engineering of adenovirus vector by placing E1A gene under CEA promoter for tumor-specific replication, while cloning chosen therapeutic genes into the viral backbone, yielding a recombinant oncolytic adenovirus plasmid for packaging. |
| Virus Production & Purification | In Vitro Validation |
| Post cloning, large-scale production of recombinant oncolytic adenoviruses involves virus proliferation in suitable cell lines and high-titer, pure virus stock solutions obtained by purification methods for in vitro/in vivo studies. | This stage includes comprehensive in vitro testing of the viral construct, assessing viral replication kinetics, therapeutic gene expression, cytotoxicity in CEA-positive cancer/normal cell lines, and apoptosis induction to provide preliminary data on the virus's selectivity and potency. |
| Preclinical In Vivo Efficacy Studies | Toxicity & Safety Assessment |
| For in-depth validation, efficacy studies in xenograft models evaluate tumor growth inhibition, survival prolongation, and viral biodistribution, yielding robust data on tumor regression and systemic distribution to demonstrate therapeutic potential. | Efficacy studies are paired with comprehensive systemic toxicity assessments, including histological analysis of major organs (liver, kidney, spleen) to confirm minimal off-target effects and the CEA promoter-driven approach's safety profile, yielding data supporting the agent's selective action. |
| Final Deliverables | Estimated Timeframe |
|
The typical timeframe for this service ranges from 12 to 15 weeks, depending on the complexity of the therapeutic gene payloads, the number of in vivo models required, and the overall scope of the project. |
[Contact us to get more details]
What We Can Offer
The OncoVirapy™ Platform offers specialized CEA Promoter-driven Oncolytic Adenovirus services, providing end-to-end solutions to revolutionize cancer therapy. Leveraging deep expertise in oncolytic virotherapy, Creative Biolabs delivers customizable, safe, and effective solutions, adapting cutting-edge technology to specific research needs. From concept to preclinical success, our commitment to scientific rigor and client collaboration translates complex gene-virotherapy mechanisms into practical, targeted cancer therapeutics.
Key Advantages and Unique Features:
- Precision Targeting: Utilizing the CEA promoter to restrict viral replication and therapeutic gene expression to CEA-positive tumor cells, minimizing off-target effects and ensuring therapy safety.
- Synergistic Payload Design: Specializing in multi-gene combinations, enhancing apoptosis and tumor regression via synergistic action in the tumor microenvironment, outperforming single-gene strategies.
- Robust Preclinical Validation: Comprehensive in vitro/in vivo models validate efficacy and safety, with rigorous testing ensuring reliable data to accelerate clinical translation.
- Enhanced Safety Profile: Preclinical models show low toxicity to normal tissues, addressing a key challenge by reducing adverse event risks.
- Accelerated Development: Streamlined workflows, state-of-the-art facilities, and expert teams enable clients to advance R&D milestones efficiently.
[Experience the Creative Biolabs Advantage - Get a Quote Today]
Case Study
The application of CEA promoter-driven oncolytic adenoviruses in preclinical murine models and CEA-positive cell lines has significantly improved tumor-specific cytotoxicity. Published data show its promising potential for CEA-expressing cancers, highlighting the promoter's tumor-selective expression and the virus' dual action of replication-induced lysis and therapeutic gene activity.
| Oncolytic Virus Construction | |
|---|---|
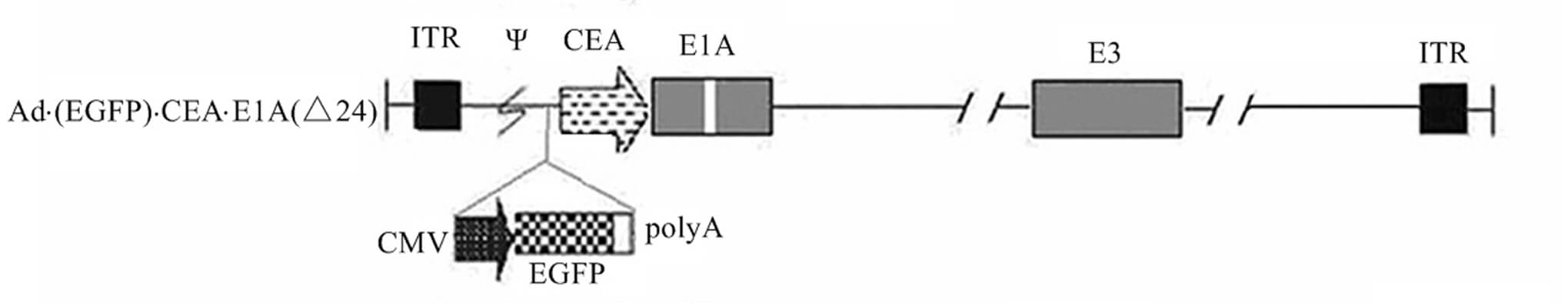
|
|
| Fig.2 Schematic representation of the oncolytic adenovirus genome obtained by replacing the native E1A promoter with the CEA promoter.2 | |
| Cell viability | Morphological |
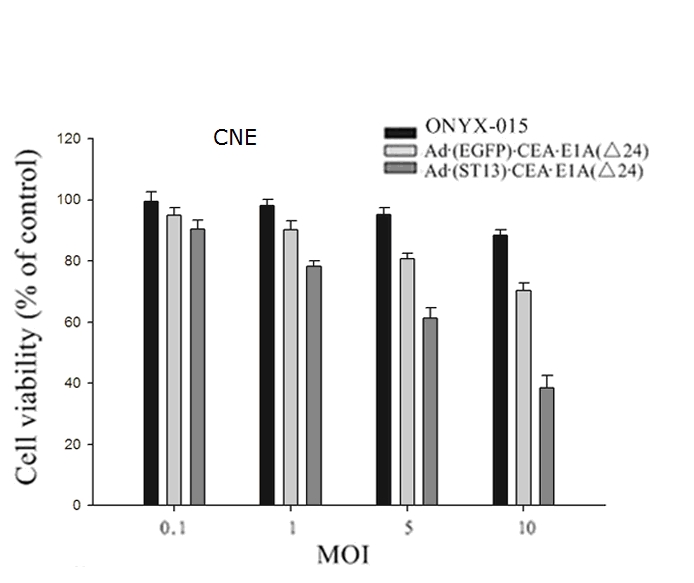
|
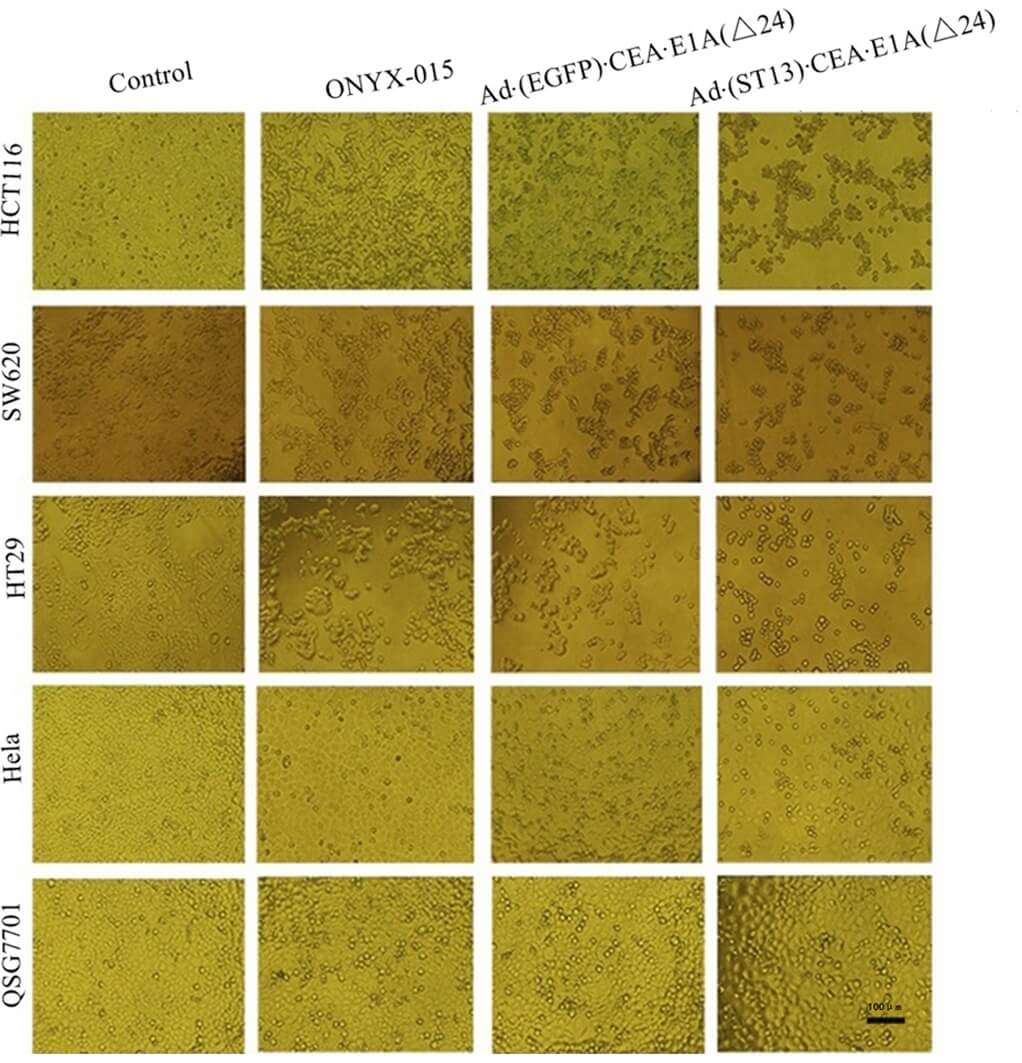
|
| Fig.2 The cytotoxicity of CEA promoter-driven oncolytic adenovirus to tumor cells was determined using MTT assay.2 | Fig.3 Morphological changes in cells infected with CEA-driven oncolytic adenovirus were observed by microscopy.2 |
| Apoptosis | |
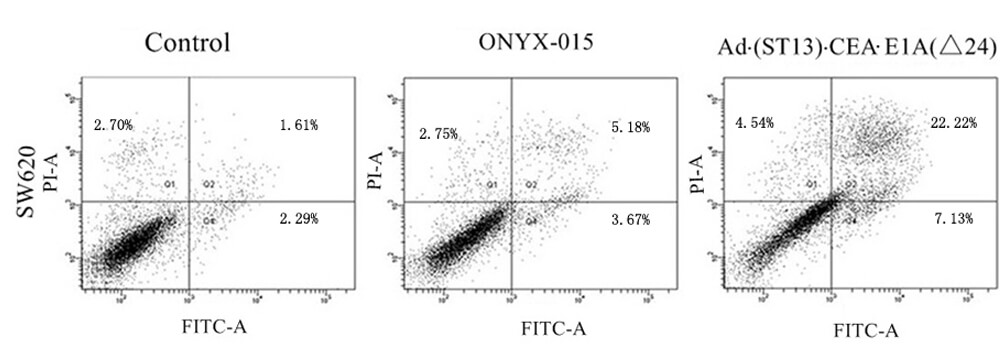
|
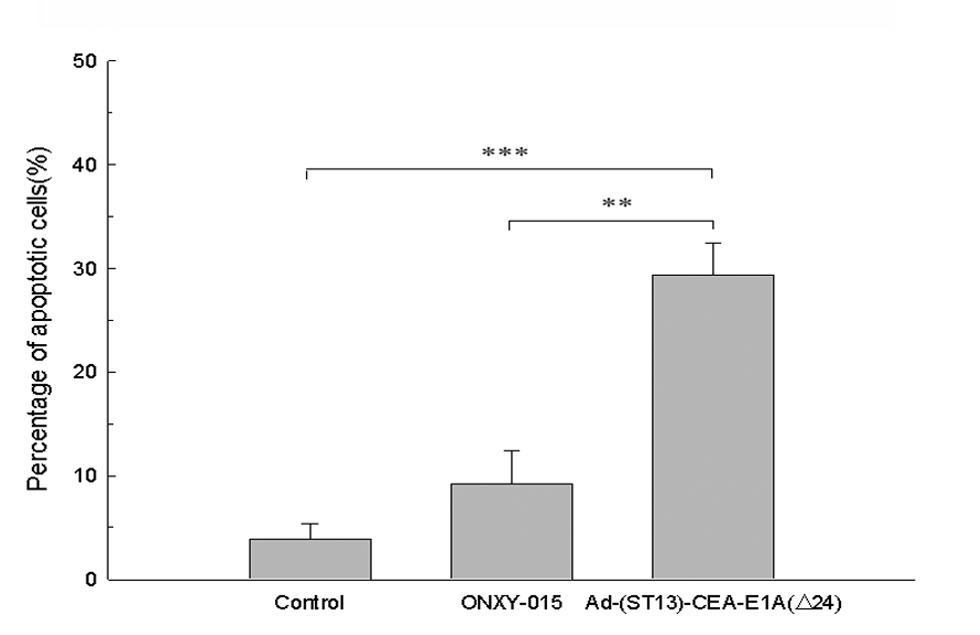
|
| Fig.4 Statistical data on the percentage of apoptotic cells.2 | Fig.5 Statistical data on the percentage of apoptotic cells.2 |
| Survival Curve | Tumor Volume |
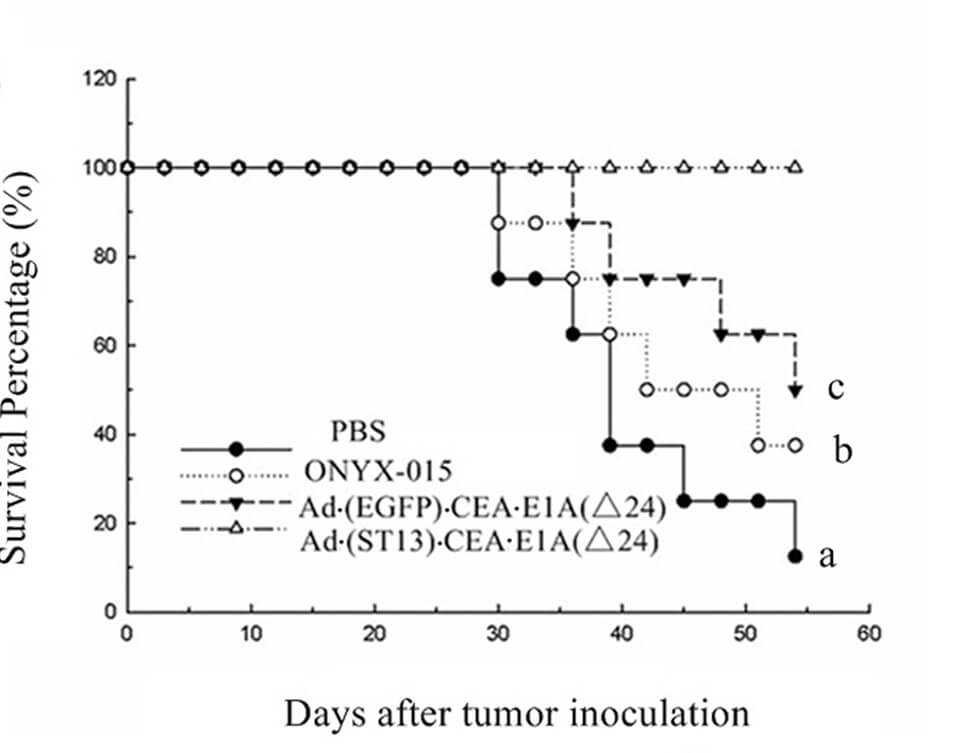
|
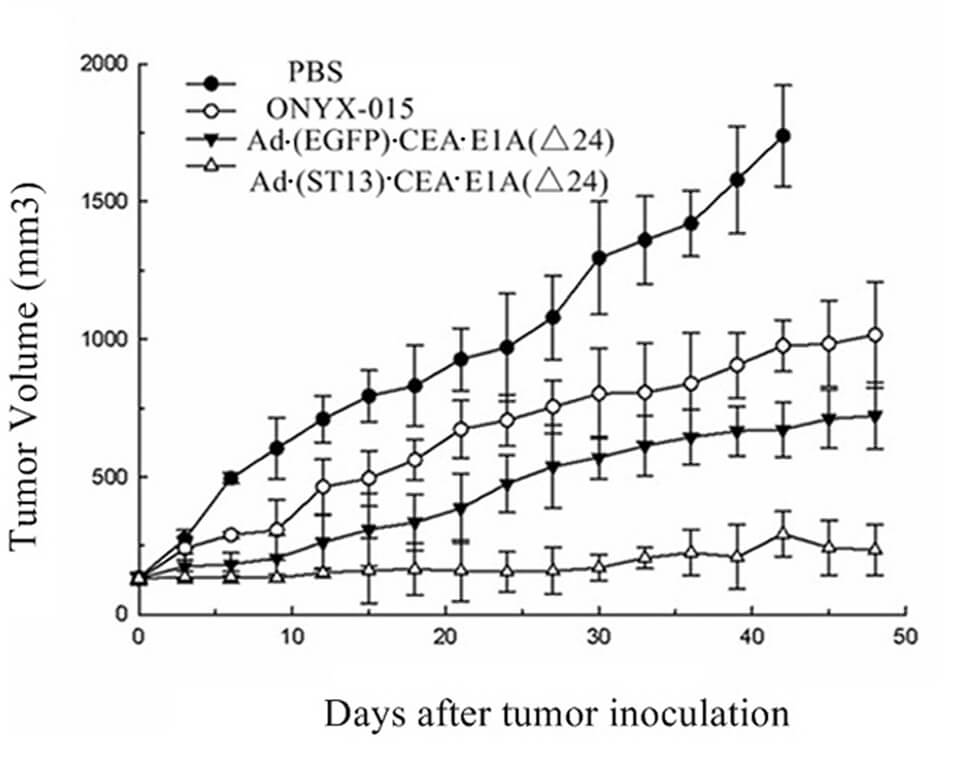
|
| Fig.6 CEA promoter-driven oncolytic adenovirus significantly prolonged the survival time of tumor-bearing mice.2 | Fig.7 CEA promoter-driven oncolytic adenovirus significantly slowed tumor growth in mice.2 |
Customer Reviews
-
"Using Creative Biolabs' CEA Promoter-driven Oncolytic Adenovirus in our research has significantly improved our ability to achieve potent and selective tumor cell killing, far surpassing the efficacy of single-gene approaches."
- Three Months Ago, J. Smi***
-
"The tumor specificity provided by Creative Biolabs' CEA promoter system is exceptional. We observed virtually no off-target effects in healthy tissues, a critical advantage over conventional therapies. This directly translated to more reliable and interpretable in vivo data."
- Last Quarter, K. Wils***
-
"Creative Biolabs' comprehensive workflow, from vector design to in vivo assessment, significantly accelerated our preclinical development. Their detailed reports and robust data allowed us to confidently advance our project, saving valuable time and resources."
- Recently, M. Cho***
FAQs
What types of cancers can benefit from CEA Promoter-driven Oncolytic Adenovirus therapy?
A: This technology is primarily designed for cancers that overexpress CEA, such as lung adenocarcinoma, colorectal cancer, gastric carcinoma, and other CEA-positive solid tumors. Our approach offers a highly targeted solution for these specific indications, allowing for precise intervention where it's most needed.
How does this therapy ensure minimal toxicity to healthy cells?
A: The key is the CEA promoter, which specifically activates viral replication and therapeutic gene expression only within CEA-positive cancer cells. This inherent tumor selectivity significantly reduces off-target effects and toxicity to healthy tissues, as demonstrated in our preclinical studies. It's a precision tool designed to spare healthy cells.
Can I incorporate my therapeutic genes into the oncolytic adenovirus?
A: Our platform is highly customizable. We can engineer the oncolytic adenovirus to carry your therapeutic gene of interest, allowing for tailored solutions to meet your specific research objectives. We encourage you to contact us to discuss your novel gene payloads and how we can integrate them into our proven system.
What is the expected timeline from project initiation to receiving results?
A: The typical timeframe for our CEA Promoter-driven Oncolytic Adenovirus service ranges from 12 to 15 weeks, depending on the complexity of the gene payload and the scope of in vitro and in vivo studies required. Our dedicated team works efficiently to provide comprehensive data promptly, keeping your project on track.
How does Creative Biolabs' CEA Promoter-driven Oncolytic Adenovirus compare to other targeted cancer therapies?
A: Our technology uniquely combines direct oncolysis with targeted gene delivery for synergistic effects, precisely targeting cancer cells, minimizing systemic side effects, and inducing anti-tumor immunity-a multi-pronged approach for superior outcomes.
Creative Biolabs CEA Promoter-driven Oncolytic Adenovirus service offers a cutting-edge solution for targeted cancer therapy, combining tumor-specific viral replication and potent gene delivery to revolutionize preclinical oncology research. Our expertise in this advanced field provides a unique advantage for your therapeutic development efforts.
[Contact Our Team to Discuss Your Project]
Related Sections
References
- Jiang, Chunyang, et al. "The indicative value of serum tumor markers for metastasis and stage of non-small cell lung cancer." Cancers 14.20 (2022): 5064. DOI: 10.3390/cancers14205064. Distributed under Open Access license CC BY 4.0, without modification.
- Zhou, Xiumei, et al. "Potent and specific antitumor effect for colorectal cancer by CEA and Rb double regulated oncolytic adenovirus harboring ST13 gene." (2012): e47566. DOI: 10.1371/journal.pone.0047566. Distributed under Open Access license CC BY 4.0, the figures were cropped.
