E2F Promoter driven Oncolytic Adenovirus Construction Service
Introduction
Creative Biolabs' E2F Promoter-driven Oncolytic Adenovirus Service revolutionizes cancer therapy with specific, potent anti-tumor solutions. Engineered for selective replication in pRB-deregulated cancer cells, our adenoviruses lyse tumors while sparing healthy tissues.
[Discover How We Can Help - Request a Consultation Today]
E2F Promoter-driven Oncolytic Adenovirus
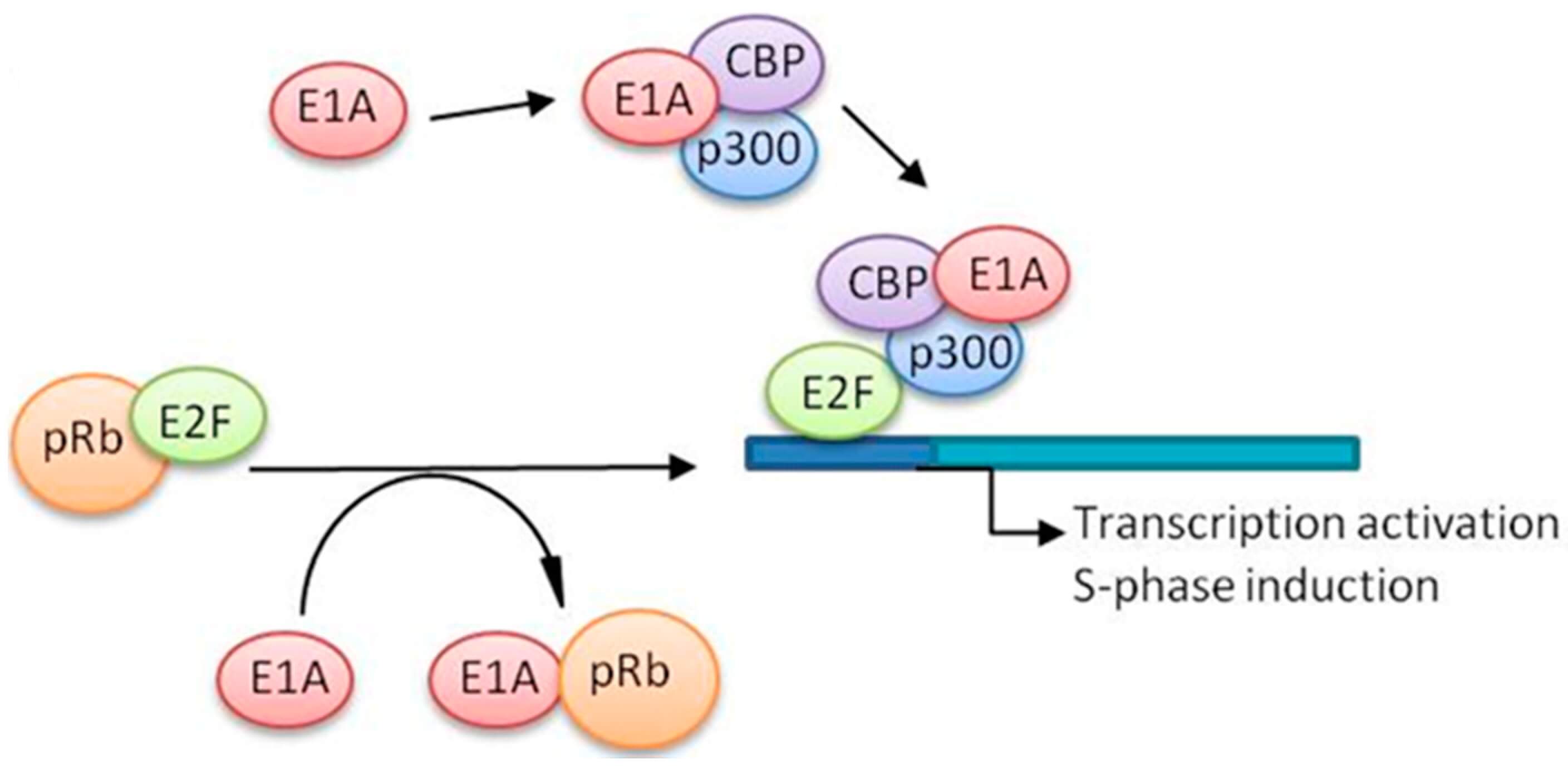 Fig.1 Schematic representation of the possible involvement of E2F in cell cycle regulation.1,3
Fig.1 Schematic representation of the possible involvement of E2F in cell cycle regulation.1,3
E2F transcription factors regulate cell cycle progression, DNA synthesis, and apoptosis. In normal cells, pRB binds E2F to repress cell division genes. However, most human cancers feature deregulated pRB pathways, leading to uncontrolled E2F activation that drives malignant proliferation. Early research into adenovirus E1A-mediated gene control revealed how viral proteins exploit E2F for replication.
Principle
The core principle behind E2F Promoter-driven Oncolytic Adenoviruses lies in their ability to exploit the specific molecular vulnerabilities of cancer cells. These viruses are engineered such that the expression of their essential early genes, particularly E1A and E4, is placed under the transcriptional control of the human E2F1 promoter.
In normal cells with an intact pRB pathway:
- pRB remains active and binds to E2F.
- This binding represses the E2F1 promoter, preventing or severely limiting the expression of the viral E1A and E4 genes.
- Without sufficient E1A and E4 expression, the viral life cycle cannot proceed efficiently, leading to significantly attenuated replication and minimal toxicity to healthy cells.
In cancer cells with a disrupted pRB pathway and deregulated E2F activity:
- The pRB-mediated repression of E2F is lost.
- This allows the E2F1 promoter to be highly active, driving robust expression of the viral E1A and E4 genes.
- The robust expression of these essential early genes enables efficient viral replication, leading to oncolysis and the release of new viral progeny that can infect and destroy adjacent tumor cells, amplifying the therapeutic effect within the tumor mass.
Furthermore, some advanced oncolytic adenoviruses might also incorporate strategies to modulate the tumor microenvironment (e.g., by expressing therapeutic cytokines) to enhance anti-tumor immune responses, providing a dual mechanism of action.
Workflow
| Required Starting Materials | Initial Consultation & Project Design |
|---|---|
|
Scientists engineer oncolytic adenovirus vectors by integrating E2F1 promoter for pRB dependency. Post-engineering, large-scale production, purification, and QC ensure high-titer, pure vectors with genomic integrity for immediate use. |
| Vector Production | In Vitro Characterization |
| Following vector engineering with E2F1 promoter integration and E1A-CR2 deletion, we conduct large-scale viral production, purification, and rigorous QC to ensure high-titer, pure vectors with intact genomes. | Through conduct in vitro assays on tumor/normal cell lines to evaluate viral replication, tumor-selective cytotoxicity, gene expression (E1A/E4), and host cell cycle. Results provide robust data on the virus' oncolytic selectivity and potency. |
| In Vivo Efficacy & Safety Evaluation | Data Analysis & Strategic Recommendations |
| We administer oncolytic adenoviruses systemically, monitoring tumor growth inhibition, survival, and intratumoral replication. Safety assessments include body weight, liver enzymes, and organ histology to confirm low systemic toxicity, providing in vivo proof-of-concept. | Our bioinformatics and oncology specialists meticulously analyze all data, providing comprehensive reports with raw data, results, statistics, and figures. Leveraging decades of expertise, we also offer strategic recommendations for preclinical optimization or clinical development. |
| Final Deliverables | Estimated Timeframe |
|
The typical timeframe for this service ranges from 8 to 14 weeks, depending on the complexity of the viral construct, the inclusion of optional preclinical evaluation, and the specific requirements of your project. More intricate designs or extensive in vivo studies may extend the duration. |
[Contact us to get more details]
What we can offer
our service is designed to provide comprehensive, custom-tailored solutions for your cancer research and therapeutic development. Our expertise ensures you receive a product and a partnership that drives success:
- Custom Vector Design & Engineering: Genetic modification of adenoviral vectors, including precise E2F promoter integration and specific deletions (e.g., E1A-CR2), optimized for your target cancer type and therapeutic objectives.
- Scalable Viral Production & Purification: From lab-scale proof-of-concept to large-scale manufacturing, delivering high-titer, high-purity oncolytic adenoviruses for rigorous preclinical evaluation.
- Rigorous In Vitro Validation: Comprehensive assays for tumor-selective replication, cytotoxicity, viral gene expression, and host cell cycle impact across cancer/normal cell lines to ensure therapeutic specificity.
- Robust In Vivo Efficacy & Safety Assessment: Preclinical studies in animal models to validate anti-tumor potency, systemic safety (reduced off-target toxicity), and viral biodistribution—critical for regulatory submissions.
- Flexible Customization: Highly tailored service packages adapted to your project needs-from target identification and lead optimization to full preclinical validation.
[Experience the Creative Biolabs' Advantage - Get a Quote Today]
Case Study
The use of E2F promoter-driven oncolytic adenoviruses in preclinical models and pRB-deregulated cancer cell lines enhances tumor-selective cytotoxicity. Studies validate its broad potential for solid tumors, emphasizing the promoter's reliance on the universal pRB pathway defect in cancer.
| Western Blot | Cell Viability |
|---|---|
|
|
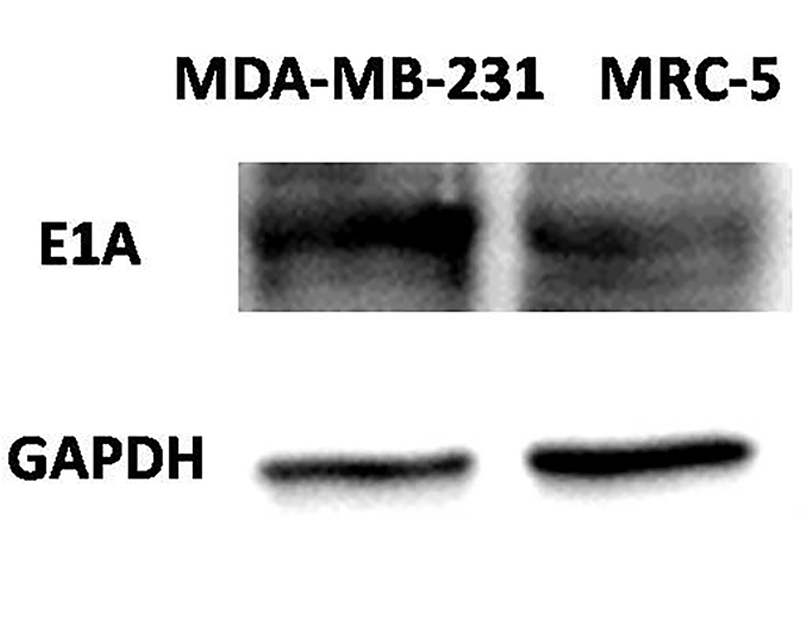
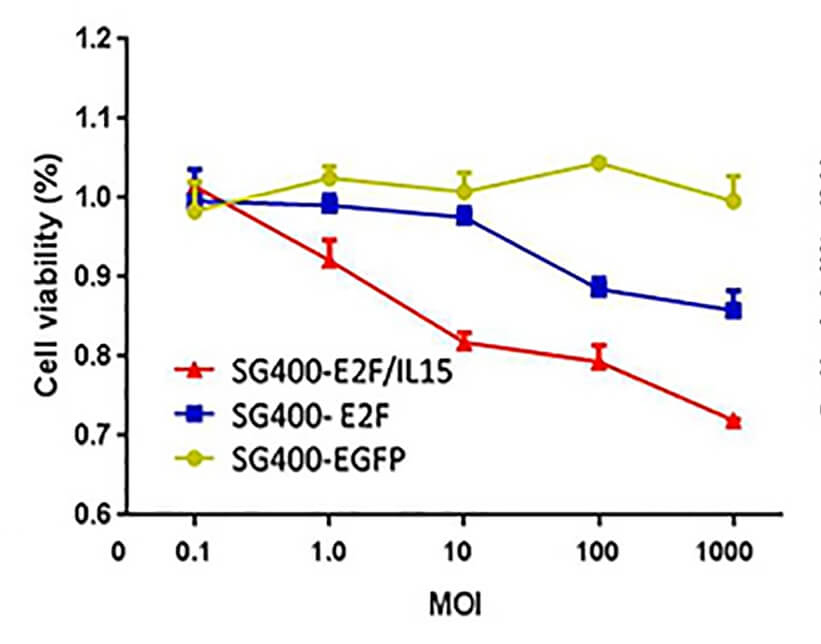
| Fig.2 E1A expression in tumor cells infected with an oncolytic adenovirus driven by the E2F promoter.2,3 | Fig.3 MTT assay is used to detect the effect of oncolytic adenovirus on the activity of tumor cells.2,3 |
| Cytotoxicity | Tumor Volume |
|
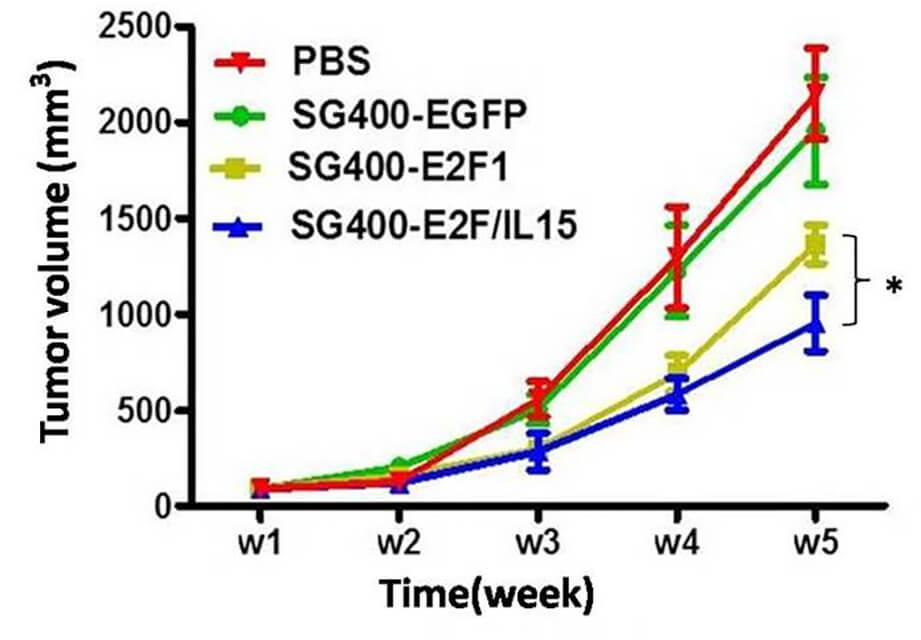
|
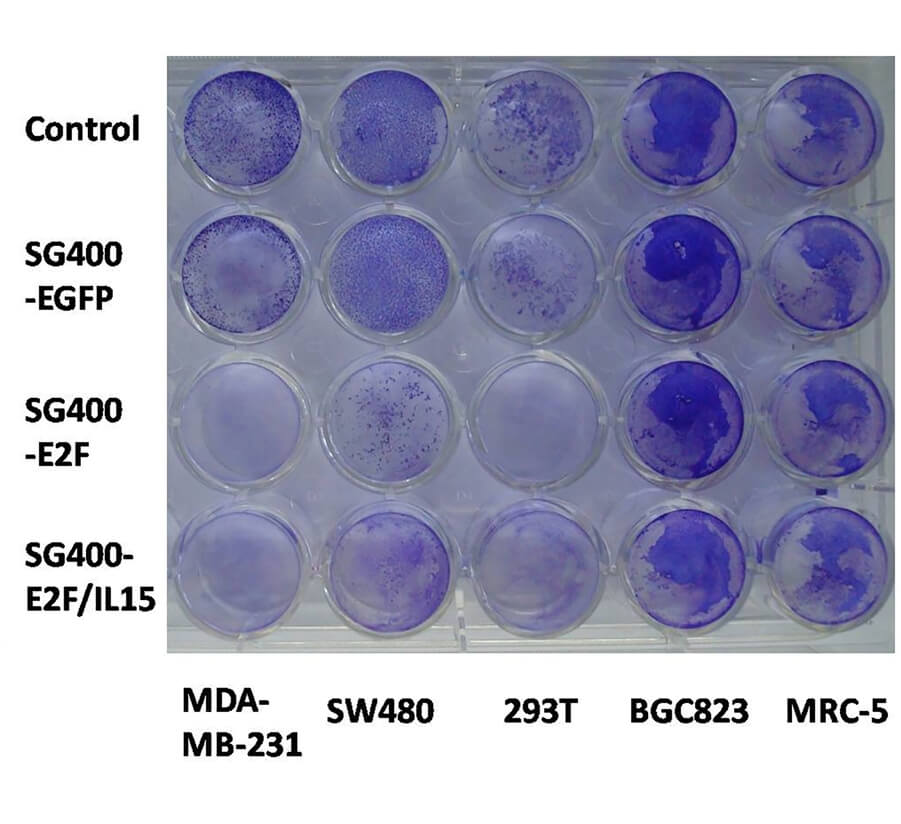
| Fig.4 Cytotoxicity is assessed by crystal violet staining.2,3 | Fig.5 An E2F promoter-driven oncolytic adenovirus retards tumor growth in mice.2,3 |
| Cell Cycle | |
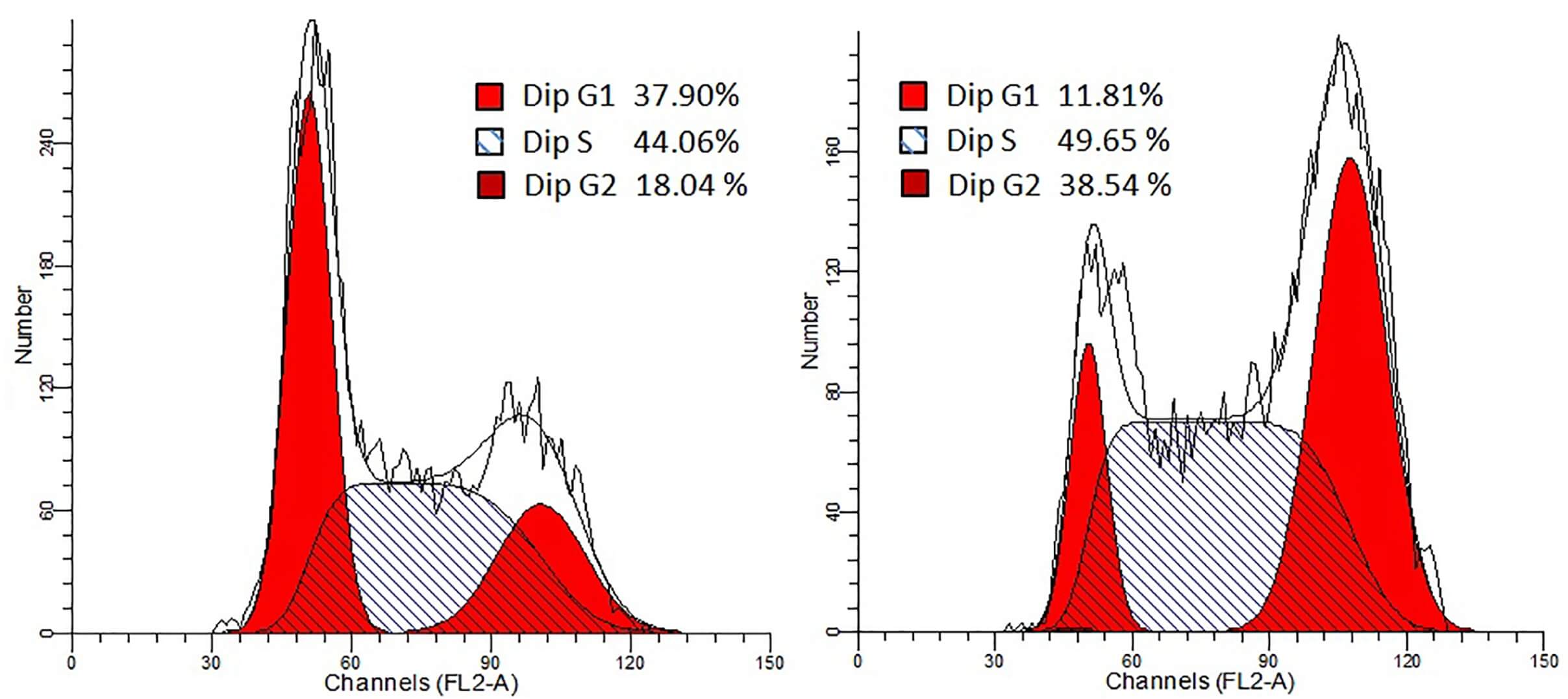
|
|
| Fig.6 The effect of E2F promoter-driven oncolytic adenovirus on tumor cell cycle was determined using flow cytometry.2,3 | |
[Contact Our Team to Discuss Your Project]
FAQs
Q: Which types of cancers are most suitable for treatment with E2F Promoter-driven Oncolytic Adenoviruses?
A: Our services are designed to target virtually any human cancer with a disrupted retinoblastoma (pRB) pathway, which includes the vast majority of solid tumors. This broad applicability is a significant advantage over many therapies.
Q: Can Creative Biolabs' E2F Promoter-driven Oncolytic Adenoviruses be used as a monotherapy or in combination with other treatments?
A: Our oncolytic adenoviruses work as potent monotherapies via direct cancer cell lysis. Their tumor-selective replication also makes them ideal for combinations-synergizing with chemo, radiation, or immune checkpoint inhibitors to boost anti-tumor effects.
Q: What are the key advantages of E2F promoter control compared to other tumor-specific promoters or viral modifications?
A: The key advantage of E2F promoter control is leveraging the universal pRB pathway deregulation in cancer. Unlike tissue-specific promoters, E2F-driven selectivity enables broad tumor applicability and robust replication, ensuring a superior therapeutic index.
Q: Are there any specific precautions or potential challenges associated with the use of E2F Promoter-driven Oncolytic Adenoviruses?
A: Though designed for low systemic toxicity, biological therapy considerations include pre-existing anti-adenoviral immunity and optimal delivery for tumor transduction. Our preclinical testing and viral immunology expertise mitigate these challenges.
Q: How do E2F Promoter-driven Oncolytic Adenoviruses compare to other viral vectors or non-viral gene therapies?
A: E2F promoter-driven oncolytic adenoviruses excel over non-replicating vectors/gene therapies via self-amplification in tumors. Unlike single-payload delivery, they continuously produce virions to spread and amplify anti-tumor effects. Targeting core oncogenic pathways, this approach offers superior precision and safety versus non-specific treatments.
Creative Biolabs leads in advanced oncolytic virotherapies. Our E2F Promoter-driven Oncolytic Adenovirus Service demonstrates scientific innovation, offering precise, safe, and effective cancer targeting. By leveraging cancer cell biological defects, we enhance tumor selectivity, reduce systemic toxicity, and unlock new therapeutic possibilities.
[Connect for an in-depth discussion]
Related Sections
References
- Baker, Alexander T., et al. "Designer oncolytic adenovirus: coming of age." Cancers 10.6 (2018): 201. DOI: 10.3390/cancers10060201. Distributed under Open Access license CC BY 4.0, without modification.
- Yan, Yang, et al. "Inhibition of breast cancer cells by targeting E2F-1 gene and expressing IL15 oncolytic adenovirus." Bioscience Reports 39.7 (2019): BSR20190384. DOI: 10.1042/BSR20190384. Distributed under Open Access license CC BY 4.0, the .figures were cropped.
