PSA Promoter driven Oncolytic Adenovirus Construction Service
Introduction
Creative Biolabs' PSA Promoter-driven Oncolytic Adenovirus service addresses challenges in developing targeted cancer treatments, overcoming off-target toxicity, and achieving superior efficacy for advanced-stage cancers. Using advanced viral engineering and transcriptional targeting, we accelerate the development of next-generation therapeutics, delivering precisely engineered viral vectors for optimal tumor specificity and potent oncolytic activity, particularly for prostate and other solid tumors harnessing PSA promoter activity. Our OncoVirapy™ Platform translates complex biology into powerful solutions, providing constructed viral agents, comprehensive efficacy data, and robust safety profiles to advance preclinical and clinical programs.
[Discover How We Can Help - Request a Consultation]
PSA Promoter-driven Oncolytic Adenovirus
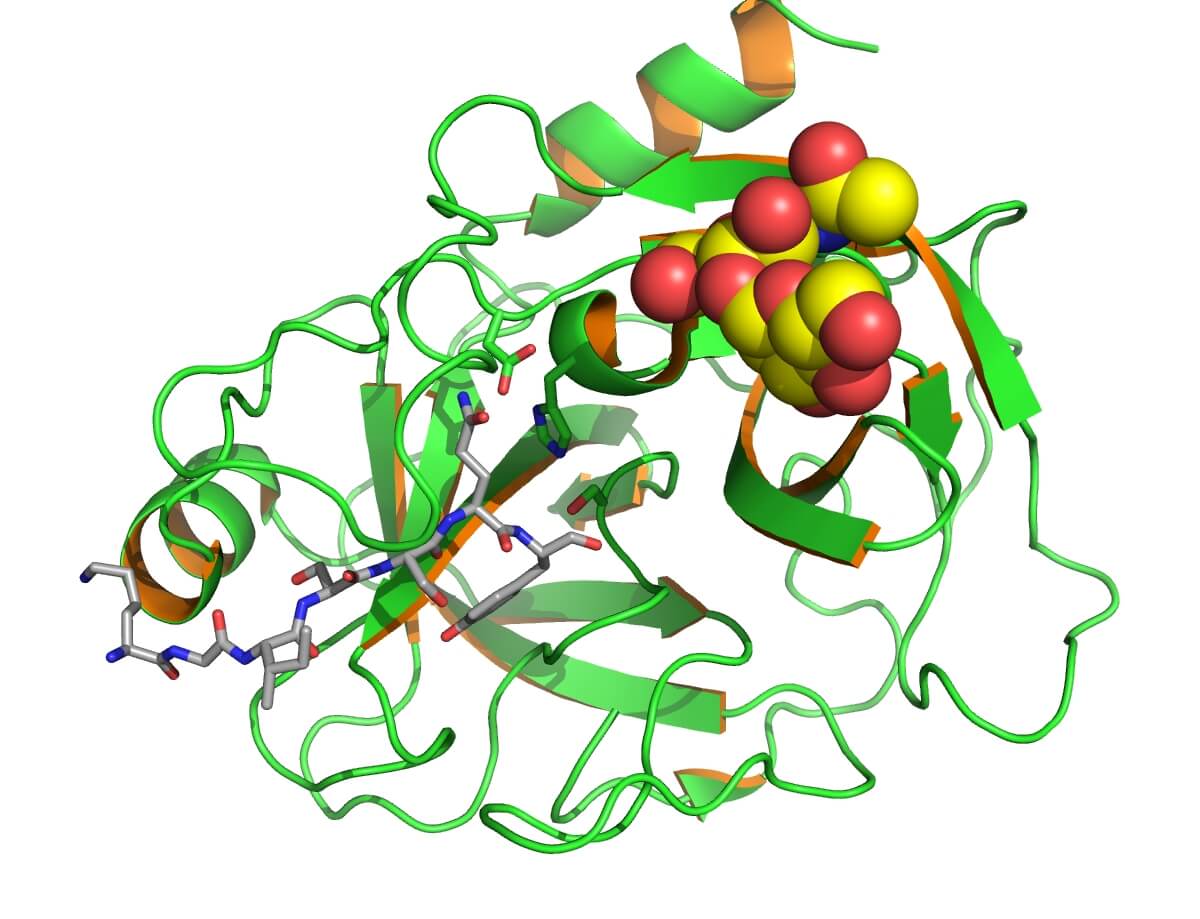 Fig.1 Human prostate-specific antigen (PSA/KLK3) with bound substrate from the complex with antibody.Distributed under CC BY-SA 3.0, from Wiki, without modification
Fig.1 Human prostate-specific antigen (PSA/KLK3) with bound substrate from the complex with antibody.Distributed under CC BY-SA 3.0, from Wiki, without modification
Prostate-specific antigen (PSA), a serine protease secreted by prostate epithelial cells, is mainly in prostate tissue and semen. Normal serum PSA is typically <4 ng/mL, but it rises in prostate diseases like cancer, BPH, or prostatitis, serving as an important marker for screening and monitoring. It's used for prostate cancer screening (with DRE), and efficacy monitoring, but lacks specificity, needing f/t PSA ratio and other tests for diagnosis.
Principle
The core principle revolves around transcriptional targeting. The oncolytic adenovirus is engineered such that its viral replication genes (e.g., E1A, E1B, essential for viral propagation and cell lysis) are placed under the control of the PSA promoter. When this engineered virus enters the body, the PSA promoter is primarily activated within prostate cancer cells, leading to:
- Selective Replication: The virus replicates efficiently only in prostate cancer cells where the PSA promoter is active, sparing healthy cells with silent/low PSA promoter activity.
- Tumor-Specific Oncolysis: Viral replication in cancer cells overwhelms cellular machinery, causing lysis and release of new virions that infect neighboring cancer cells, propagating the oncolytic effect.
- Immune Activation: Cancer cell lysis exposes tumor antigens, stimulating the host immune system to mount an anti-tumor response for long-term efficacy.
Furthermore, these viruses can be "armed" with additional therapeutic genes whose expression is also driven by the PSA promoter, amplifying the therapeutic effect specifically within the tumor.
Advantages
- High Tumor Selectivity: The primary advantage is its exceptional specificity for prostate cancer cells, driven by the unique activity of the PSA promoter. This dramatically reduces off-target toxicity, a major limitation of conventional chemotherapy and radiotherapy.
- Reduced Systemic Toxicity: By minimizing infection and replication in healthy tissues, the risk of systemic side effects such as hepatotoxicity (liver damage) is significantly reduced compared to non-targeted viral therapies or chemotherapy.
- Immune System Engagement: The oncolytic process releases danger signals and tumor antigens, which can activate both innate and adaptive immune responses against the tumor, potentially leading to durable anti-tumor immunity.
- High Transduction Efficiency: Adenoviruses are known for their high efficiency in delivering genetic material into a wide range of cell types, including many cancer cells.
Workflow
| Required Starting Materials | Project Consultation & Design |
|---|---|
|
We start with a tailored consultation to understand your goals, then engineer a bespoke PSA-driven oncolytic adenovirus with a project plan. |
| Adenovirus Construction & Engineering | Viral Production & Purification |
| Using advanced recombinant DNA tech, we construct the adenovirus vector by cloning the PSA promoter to drive oncolytic/therapeutic gene expression in PSA+ cells, modifying the capsid for better targeting. | High-titer viral stocks are produced using specialized cell lines, followed by rigorous purification processes. This ensures a highly pure and potent viral product, free from contaminants, suitable for in vitro and in vivo studies. |
| Characterization & Quality Control | Preclinical Efficacy & Safety Evaluation |
| Each viral batch undergoes extensive characterization, including titration (determining viral particle and infectious unit counts), genomic integrity verification, and confirmation of PSA promoter specificity in vitro using both PSA-positive and PSA-negative cell lines. | For clients needing comprehensive data, we provide preclinical evaluations in vitro oncolysis assays, bystander effect analysis, and in vivo studies using xenograft/syngeneic models to assess tumor regression, survival, and toxicity, ensuring a complete safety and efficacy profile. |
| Final Deliverables | Estimated Timeframe |
|
The typical timeframe for this service ranges from 8 to 14 weeks, depending on the complexity of the viral construct, the inclusion of optional preclinical evaluation, and the specific requirements of your project. More intricate designs or extensive in vivo studies may extend the duration. |
[Contact us to get more details]
What we can offer
- Customized Viral Vector Design: Engineer PSA promoter-driven oncolytic adenoviruses tailored to your research, featuring specific therapeutic gene payloads, capsid modifications for enhanced tropism, and optimized oncolytic potency for selected cancer cell lines.
- Precision Transcriptional Targeting: Leverage PSA promoter specificity to ensure selective viral replication and gene expression exclusively in PSA-positive tumor cells, minimizing off-target effects.
- Optimized Viral Production & Purification: Scale-up manufacturing delivers high-titer, high-purity adenoviruses suitable for in vitro/in vivo applications.
- Rigorous Quality Control & Characterization: Each batch undergoes viral titration, genomic integrity verification, and in vitro specificity assays to ensure consistency and reliability.
- Expert Consultation & Project Support: Receive end-to-end guidance from design and feasibility assessment to clinical translation strategies.
- Flexible Production Scales: Adapt production volumes to match your needs, from lab research to preclinical studies, ensuring a seamless transition from concept to application.
[Experience the Creative Biolabs Advantage - Get a Quote Today]
Case Study
The use of PSA promoter-driven oncolytic adenoviruses in preclinical models and PSA-positive cell lines boost tumor-specific killing. Research validates its potential for prostate and related cancers, highlighting the promoter's tumor selectivity and the virus's dual action of replication-induced lysis and gene-mediated cytotoxicity for precise, potent anti-tumor effects.
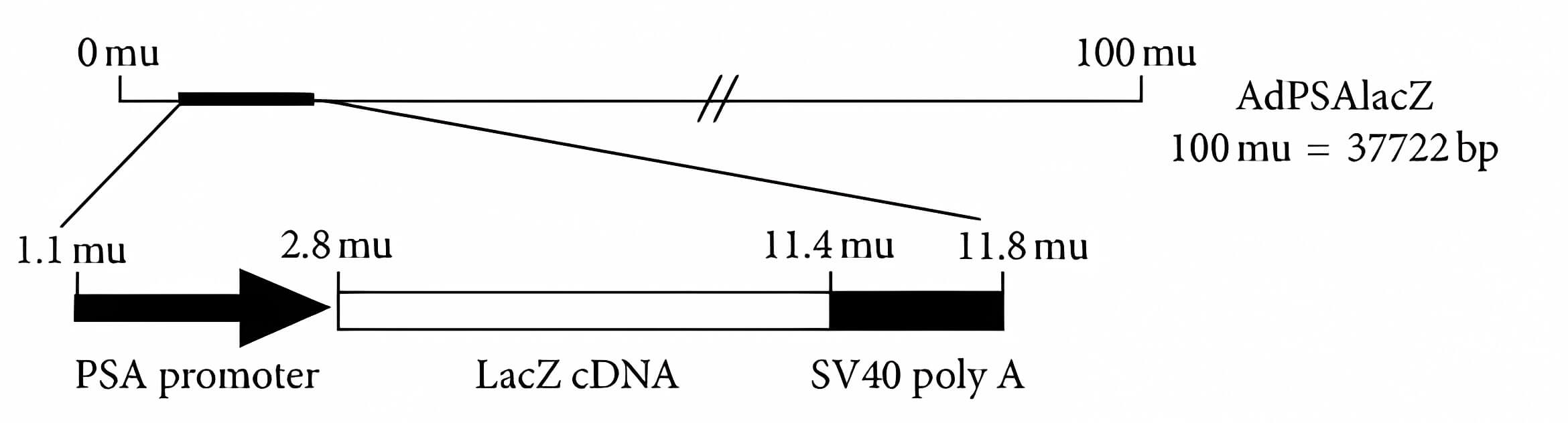
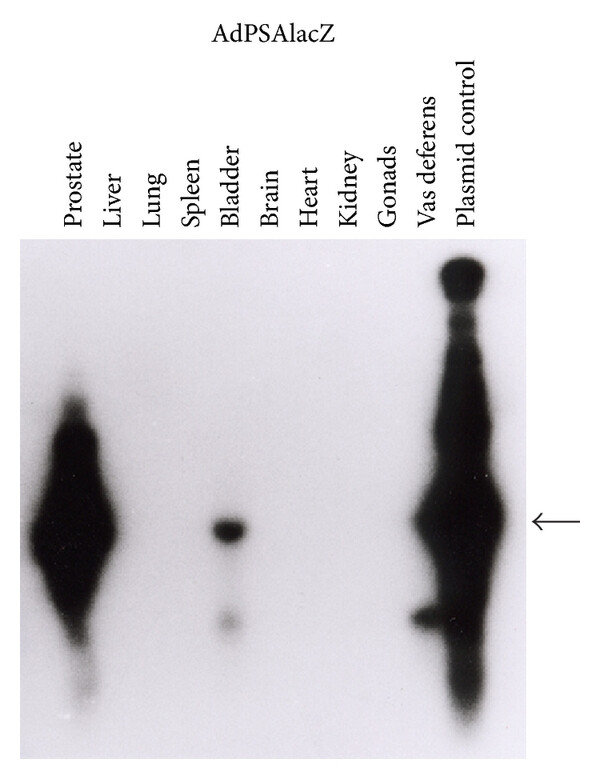
| Oncolytic Virus Construction | PSP Specificity |
|---|---|
|
|
| Fig.2 Schematic representation of the oncolytic adenovirus genome using PSA promoter-driven.1 | Fig.3 Adenoviral DNA sequence PCR Southern hybridization is used to detect specificity.1 |
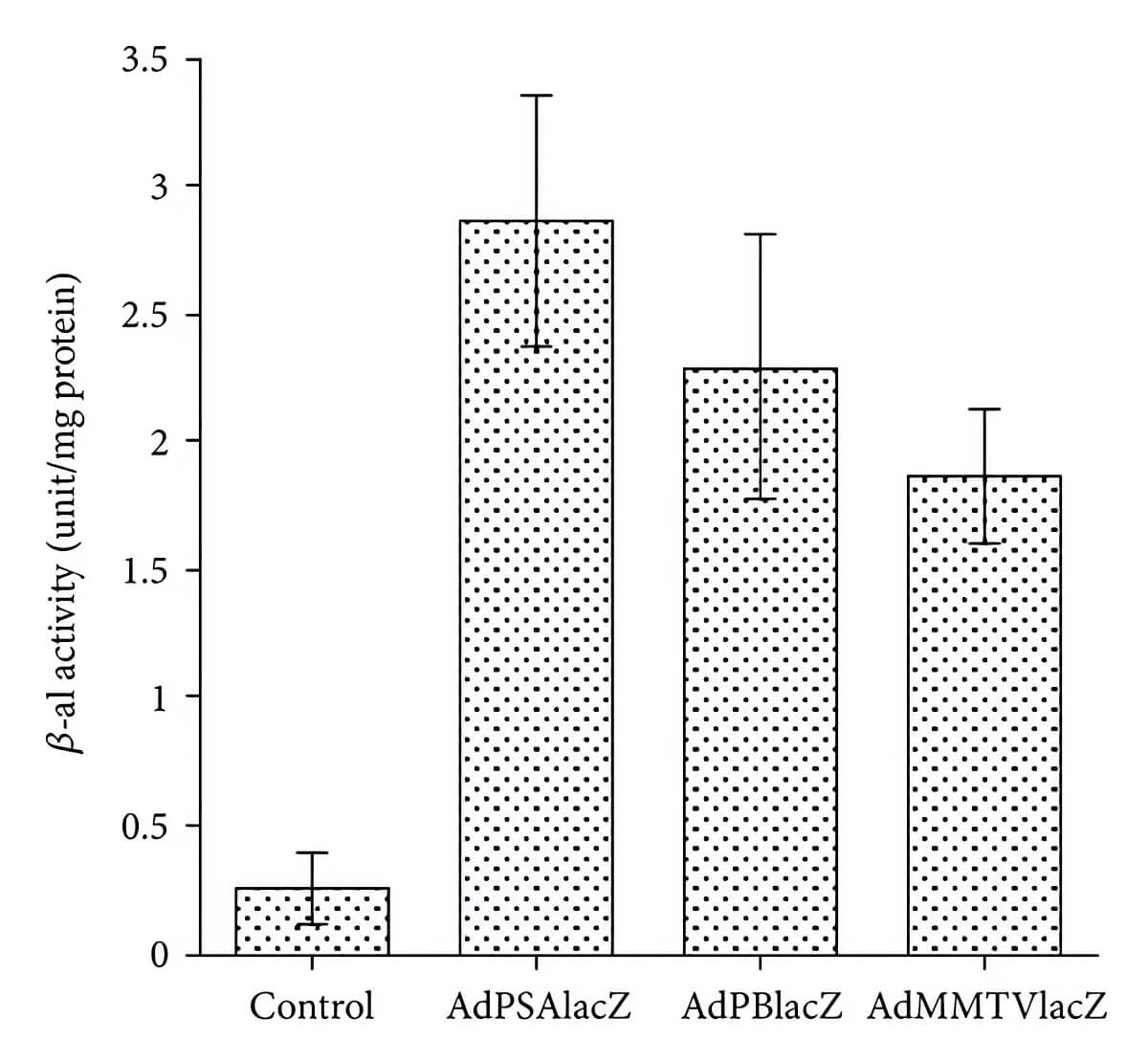
| PSP Activity | Tumor Volume |
|
|
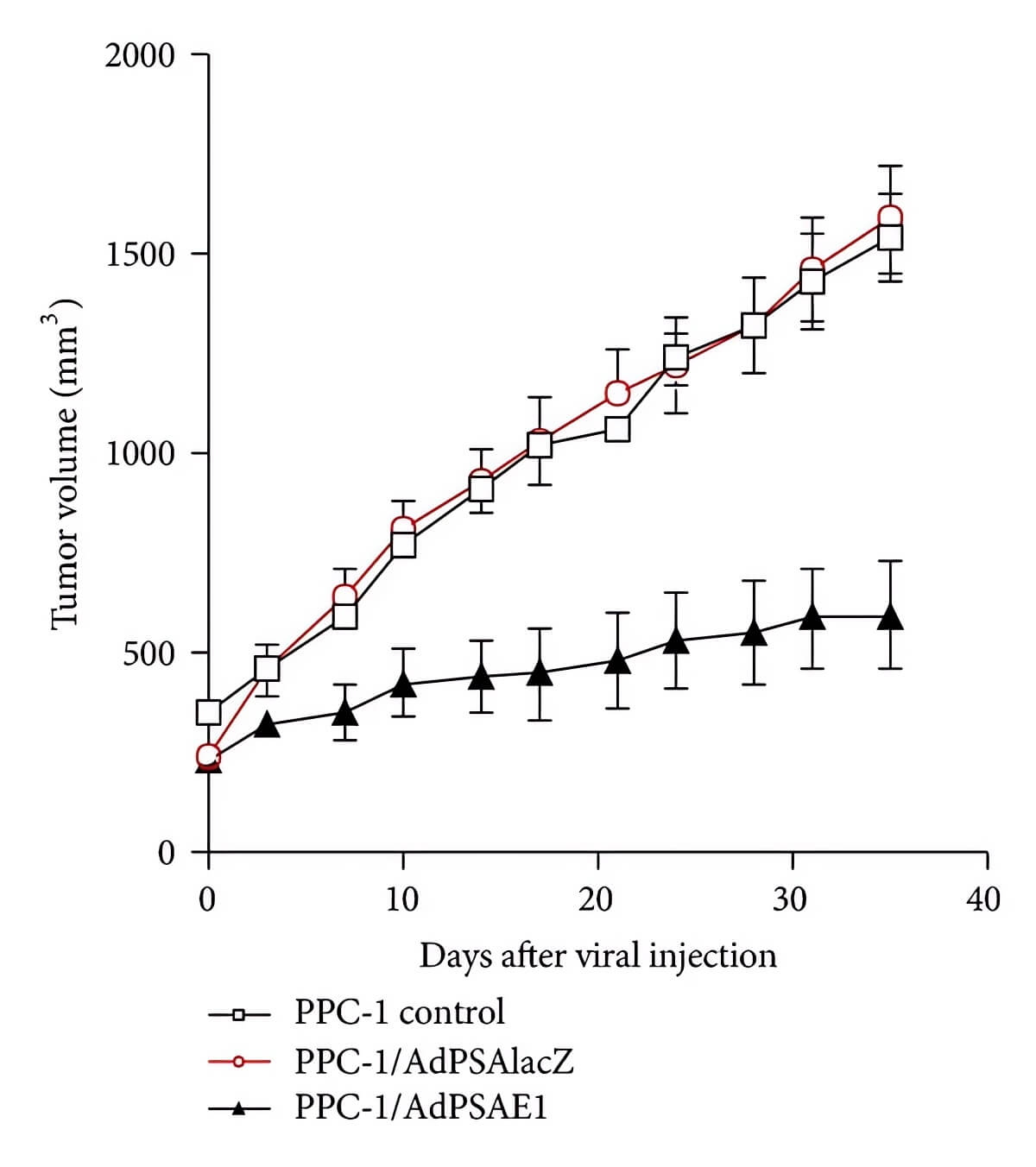
| Fig.4 β-galactosidase activity is used to indicate PSP activity.1 | Fig.5 Oncolytic adenovirus with PSA-E1 expression cassette can significantly reduce tumor volume in mice.1 |
FAQs
Q: How does the PSA promoter ensure specificity, and what if my target cells have low PSA expression?
A: The PSA promoter drives oncolytic virus replication in prostate cancer cells, minimizing off-target effects. For tumors with low/heterogeneous PSA expression, our team can explore alternative targeting strategies.
Q: Can your PSA Promoter-driven Oncolytic Adenovirus be combined with other cancer therapies?
A: Our PSA-driven oncolytic adenoviruses allow engineering with additional therapeutic genes or combination with chemo/radiation/immunotherapies for synergistic anti-tumor effects.
Q: What safety precautions are in place to prevent off-target viral replication?
A: Our oncolytic adenoviruses are engineered for safety. PSA promoter control ensures tumor specificity, minimizing replication in healthy cells. Rigorous QC and additional safety mechanisms further enhance the therapeutic window.
Q: What kind of data can I expect regarding the efficacy of the oncolytic adenovirus?
A: We offer comprehensive characterization data, covering viral titer, genomic integrity, and in vitro specificity. For clients choosing our preclinical evaluation service, we provide in vitro oncolysis data and in vivo efficacy data from xenograft models, including tumor regression, survival curves, and immunohistochemistry analysis.
Q: How does Creative Biolabs' approach compare to other viral vector services or traditional gene therapy methods?
A: Creative Biolabs stands out with its expertise in engineering oncolytic adenoviruses, specializing in transcriptional targeting via promoters like PSA. Unlike generic viral vector services, we focus on highly specialized, tumor-selective agents with superior safety and efficacy. Our customized solutions and strict QC ensure products are tailored to your research needs, advancing targeted cancer therapy.
Creative Biolabs stands as your premier partner in the development of advanced oncolytic viral therapies, specifically PSA Promoter-driven Oncolytic Adenovirus. Our commitment to scientific excellence, combined with our unparalleled expertise in viral engineering and cancer biology, ensures that your projects are delivered with precision, potency, and a clear path toward clinical translation. We provide innovative, targeted solutions that empower researchers and biopharmaceutical companies to overcome the challenges of advanced cancer treatment, accelerate discovery, and ultimately bring life-changing therapies to patients.
[Contact Our Team for More Information and to Discuss Your Project]
Related Sections
Reference
- Lu, Yi, et al. "Comparison of prostate‐specific promoters and the use of PSP‐driven virotherapy for prostate cancer." BioMed Research International 2013.1 (2013): 624632. DOI: 10.1155/2013/624632. Distributed under Open Access license CC BY 3.0, without modification.
