hTERT Promoter driven Oncolytic Adenovirus Construction Service
Introduction
Creative Biolabs' hTERT Promoter-driven Oncolytic Adenovirus Construction service assists in overcoming challenges such as off-target toxicity, limited solid tumor efficacy, and resistance development in cancer therapeutic programs. Leveraging advanced viral engineering and tumor-specific promoter technology, the service delivers highly specific and potent anti-cancer therapies. Our OncoVirapy™ Platform provides expertly engineered oncolytic adenoviruses that selectively replicate in and lyse malignant cells, minimizing off-target effects and enhancing anti-tumor immunity. Tailored to meet the need for effective and safe cancer treatments, the service offers a powerful tool for preclinical validation and therapeutic discovery.
[Discover How We Can Help - Request a Consultation]
hTERT Promoter-driven Oncolytic Adenovirus
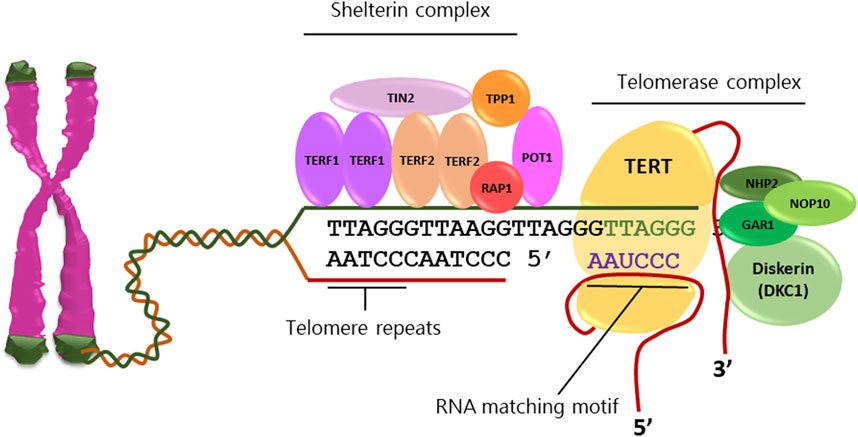 Fig.1 The individual components of telomerase and the location of TERT.1
Fig.1 The individual components of telomerase and the location of TERT.1
The hTERT promoter-driven oncolytic adenovirus is a cancer therapy strategy. It uses the hTERT promoter's differential activity: highly active in most cancer cells to drive telomerase expression, but inactive in normal somatic cells. Engineered adenoviruses place essential replication genes/therapeutic transgenes under hTERT promoter control, enabling preferential replication and therapeutic protein expression in cancer cells. This leads to selective tumor cell lysis, sparing healthy tissues and improving the therapeutic window.
Advantages
- Exceptional Tumor Selectivity: Tumor-specific hTERT promoter activity enables selective viral replication and oncolysis in cancer cells, minimizing off-target toxicity.
- Enhanced Safety Profile: Tight regulation of viral replication in malignant cells improves the therapeutic index.
- Potent Anti-Tumor Efficacy: Direct oncolysis releases tumor antigens and DAMPs, stimulating anti-tumor immune responses via a dual killing-activation mechanism.
- Broad Applicability: Effective against >85% of human cancers with hTERT overexpression, including solid tumors and glioblastoma.
- Synergy with Other Therapies: Complements chemotherapy, radiation, and immunotherapies to overcome resistance and enhance outcomes.
Workflow
| Required Starting Materials | Project Scoping & Design |
|---|---|
Target gene sequences for custom adenovirus modifications |
We start with a detailed consultation to understand your cancer model, therapeutic goals, and desired vector modifications/payloads. Our team then designs the hTERT-driven adenoviral construct for optimal tumor specificity and replication. |
| Vector Design & Production | In Vitro Characterization |
| After design approval, our scientists genetically modify the adenovirus to incorporate the hTERT promoter and specified therapeutic genes, then conduct large-scale production and rigorous purification to ensure high-quality, pure hTERT-driven oncolytic adenovirus vectors. | Comprehensive in vitro assays characterize engineered viruses, testing viral replication efficiency, cytolytic activity, and selectivity between client-provided cancer cell lines and normal cell controls. |
| Preclinical Efficacy Evaluation | Data Analysis and Reporting |
| For advanced validation, we collaborate on or conduct preclinical efficacy studies, assessing anti-tumor effects, viral biodistribution, and safety in animal models. | Upon completion of experimental work, we provide a comprehensive analysis of all generated data. This includes a detailed interpretation of results, raw data files, and a final scientific report summarizing all experimental findings and conclusions. |
| Final Deliverables | Estimated Timeframe |
Viral titer data Immunohistochemistry results Other relevant assay data as generated |
The typical timeframe for this service ranges from 10-15 weeks, depending on the complexity of the engineering required, the number of constructs to be optimized, and the scope of in vivo studies. |
[Contact us for a more detailed workflow]
What we can offer
- End-to-End Project Support
Integrated services cover design, engineering, production, and characterization for streamlined preclinical validation.
- Flexible and Scalable Vector Production
State-of-the-art facilities enable scalable production from research batches to large preclinical quantities.
- Precision Engineering and Optimization
Genetic expertise ensures hTERT promoter integration and transgene expression optimization for maximal tumor specificity.
- Rigorous Quality Control & Analytical Expertise
Stringent QbD-compliant processes with advanced assays for vector purity, titer, infectivity, and selectivity.
- Customized Vector Design & Payload Integration
Tailored modifications and payload integration (e.g., immunomodulatory genes, prodrug enzymes) for multi-modal strategies.
- Cell Line and Animal Model Optimization
Guidance in selecting/optimizing tumor cell lines and in vivo models for predictive efficacy evaluation.
- Dedicated Scientific Consultation
Expert virology/oncology support for experimental design, troubleshooting, data interpretation, and research strategy.
Case Study
The employment of hTERT promoter-driven oncolytic adenoviruses across common in vivo murine cancer models and in vitro tumor cell line systems has notably improved tumor-specific lysis efficiency. Many published studies underscore its promising therapeutic potential for cancer treatment, emphasizing the promoter's tumor-selective expression profile and the virus's dual mode.
| Construction of Oncolytic Virus | Rate of Replication |
|---|---|
|
|
|
| Cytopathic | Secretion of cytokines |
|
|
|
| Curve of survival | Tumor Volumes |
|
|
|
[Experience the Creative Biolabs Advantage - Get a Quote Today]
Customer Reviews
-
"Unparalleled Specificity! Using Creative Biolabs' hTERT Promoter-driven Oncolytic Adenovirus in our research has significantly improved the therapeutic index of our constructs, enabling us to achieve potent anti-tumor effects with remarkably reduced off-target toxicity."
[March 2025], Dr. L***a M.
-
"Game-Changing Efficacy in Solid Tumors. Creative Biolabs' hTERT platform provided the precise tumor-specific replication we needed, facilitating deep tumor penetration and robust viral spread, which was critical for our challenging pancreatic cancer models. This technology truly delivered on its promise."
[January 2025], Prof. K***n W.
-
"Accelerated Our Preclinical Development. The comprehensive support and high-quality vectors from Creative Biolabs allowed us to streamline our preclinical validation process, dramatically cutting down the time from concept to in vivo proof-of-concept for our oncolytic program. Their expertise was invaluable."
[February 2025], Dr. R***t J.
FAQs
Q1: How does hTERT targeting improve safety compared to other oncolytic virus platforms?
A1: The hTERT promoter is active in most cancer cells but quiescent in healthy tissues, enabling our oncolytic adenoviruses to replicate and act selectively in malignant cells, reducing off-target toxicity and enhancing safety. This precision is central to our design.
Q2: What types of cancer are most suitable for hTERT-driven oncolytic adenovirus therapy?
A2: Given the widespread overexpression of hTERT in human malignancies, our hTERT Promoter-driven Oncolytic Adenovirus platform is highly adaptable and suitable for a broad spectrum of cancers. This includes various solid tumors, such as those of the lung, colon, breast, prostate, and liver, as well as highly aggressive tumors like glioblastoma.
Q3: Can Creative Biolabs' hTERT platform be combined with other cancer treatments?
A3: One of the key advantages of hTERT-driven oncolytic adenoviruses is their strong potential for synergistic effects when combined with other therapeutic modalities. They can complement chemotherapy, radiation therapy, and various immunotherapies by enhancing tumor cell killing, modifying the tumor microenvironment, and amplifying anti-tumor immune responses.
Q4: How can I assess the efficacy of the hTERT-driven oncolytic adenovirus in my specific model?
A4: Creative Biolabs offers comprehensive in vitro and in vivo characterization services for hTERT-driven oncolytic adenoviruses, including viral replication, cell viability, immune response, and tumor regression assays in animal models. We provide detailed reports and data analysis.
At Creative Biolabs, our hTERT Promoter-driven Oncolytic Adenovirus service stands at the forefront of targeted cancer therapy. By leveraging the unique biological signature of cancer cells, we offer innovative, highly specific, and potent oncolytic solutions designed to accelerate your preclinical drug discovery and development. Our commitment to scientific excellence, combined with a comprehensive workflow and proven results, ensures that you receive cutting-edge tools to advance your fight against cancer.
[Contact Our Team for More Information]
Related Sections
References
- Tornesello, Maria Lina, et al. "Reactivation of telomerase reverse transcriptase expression in cancer: The role of TERT promoter mutations." Frontiers in Cell and Developmental Biology 11 (2023): 1286683. DOI: 10.3389/fcell.2023.1286683. Distributed under Open Access license CC BY 4.0, without modification.
- Osipov, Ivan D., et al. "Development of oncolytic vectors based on human adenovirus type 6 for cancer treatment." Viruses 15.1 (2023): 182. DOI: 10.3390/v15010182
- Hashimoto, Yuuri, et al. "The hTERT promoter enhances the antitumor activity of an oncolytic adenovirus under a hypoxic microenvironment." PloS one 7.6 (2012): e39292. DOI: 10.1371/journal.pone.0039292
- Distributed under Open Access license CC BY 4.0, the figures were cropped.

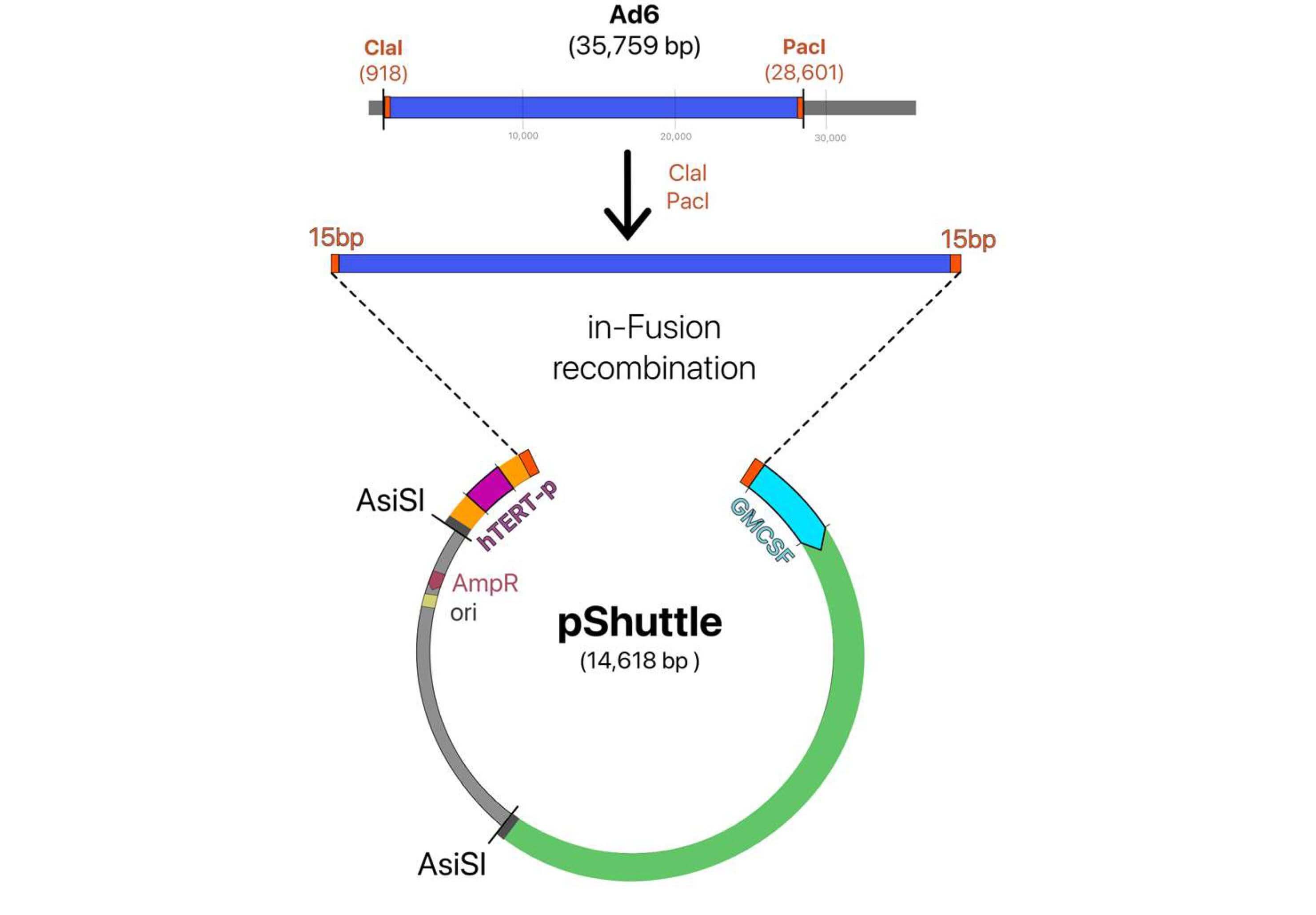 Fig.2 Construction of oncolytic adenovirus with hTERT promoter.2,4
Fig.2 Construction of oncolytic adenovirus with hTERT promoter.2,4
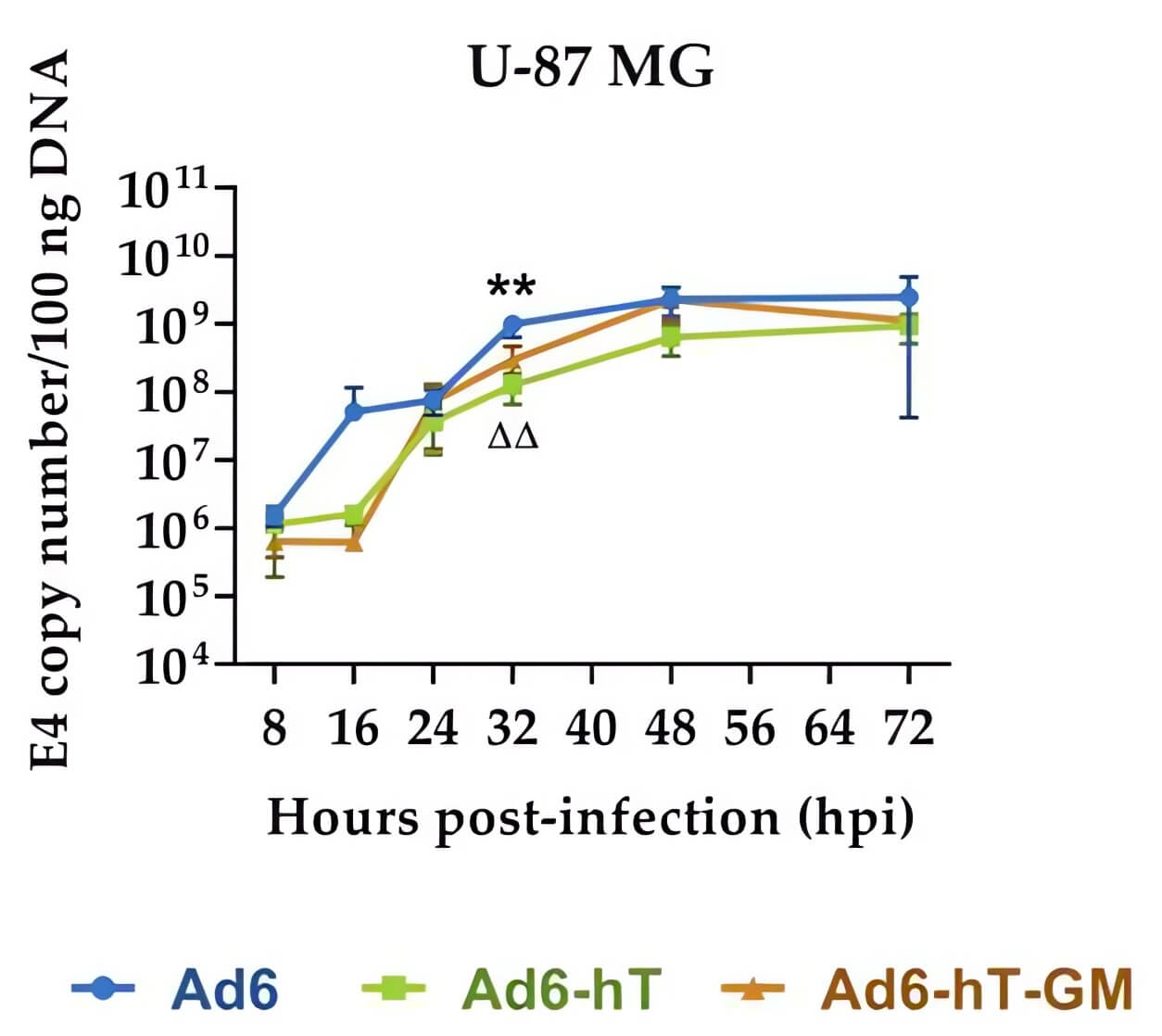 Fig.3 Effect of genomic modifications on the rate of adenovirus replication in tumor cell lines.2,4
Fig.3 Effect of genomic modifications on the rate of adenovirus replication in tumor cell lines.2,4
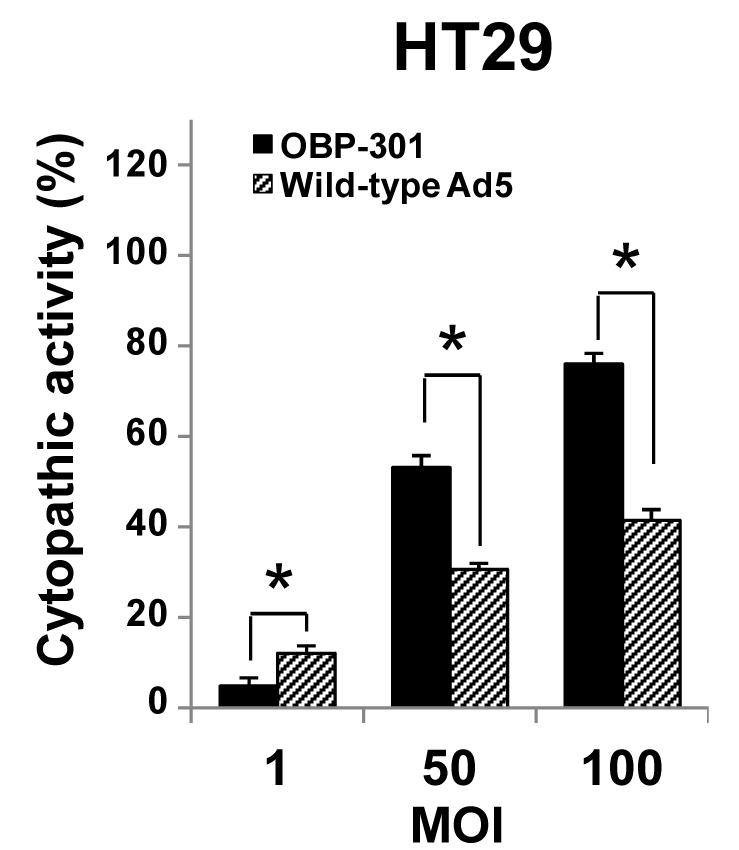 Fig.4 Cytopathic effect of adenovirus loaded with hTERT promoter and wild-type Ad5.3,4
Fig.4 Cytopathic effect of adenovirus loaded with hTERT promoter and wild-type Ad5.3,4
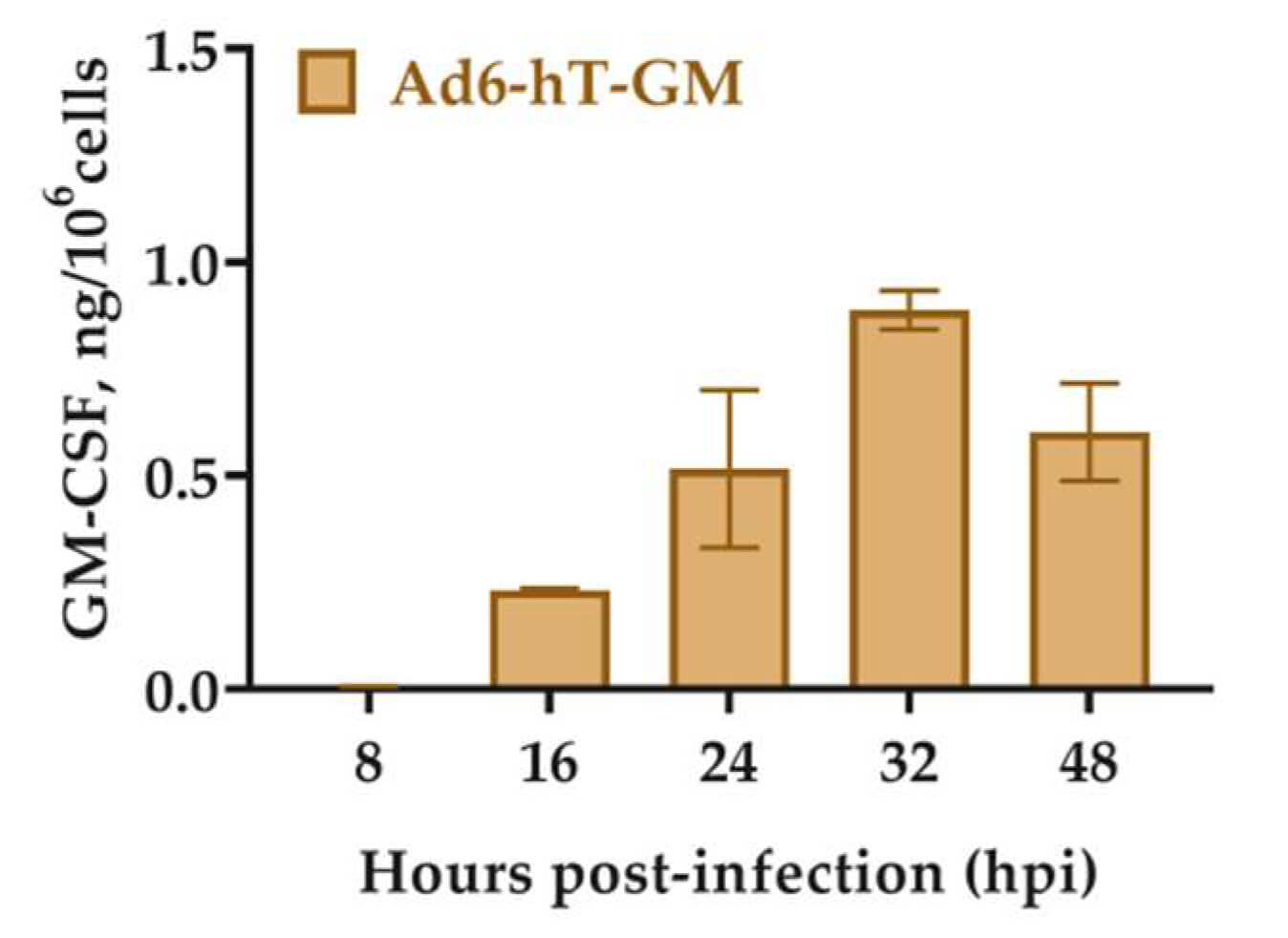 Fig.5 The ability of an oncolytic adenovirus loaded with the hTERT promoter to secrete GM-CSF.2,4
Fig.5 The ability of an oncolytic adenovirus loaded with the hTERT promoter to secrete GM-CSF.2,4
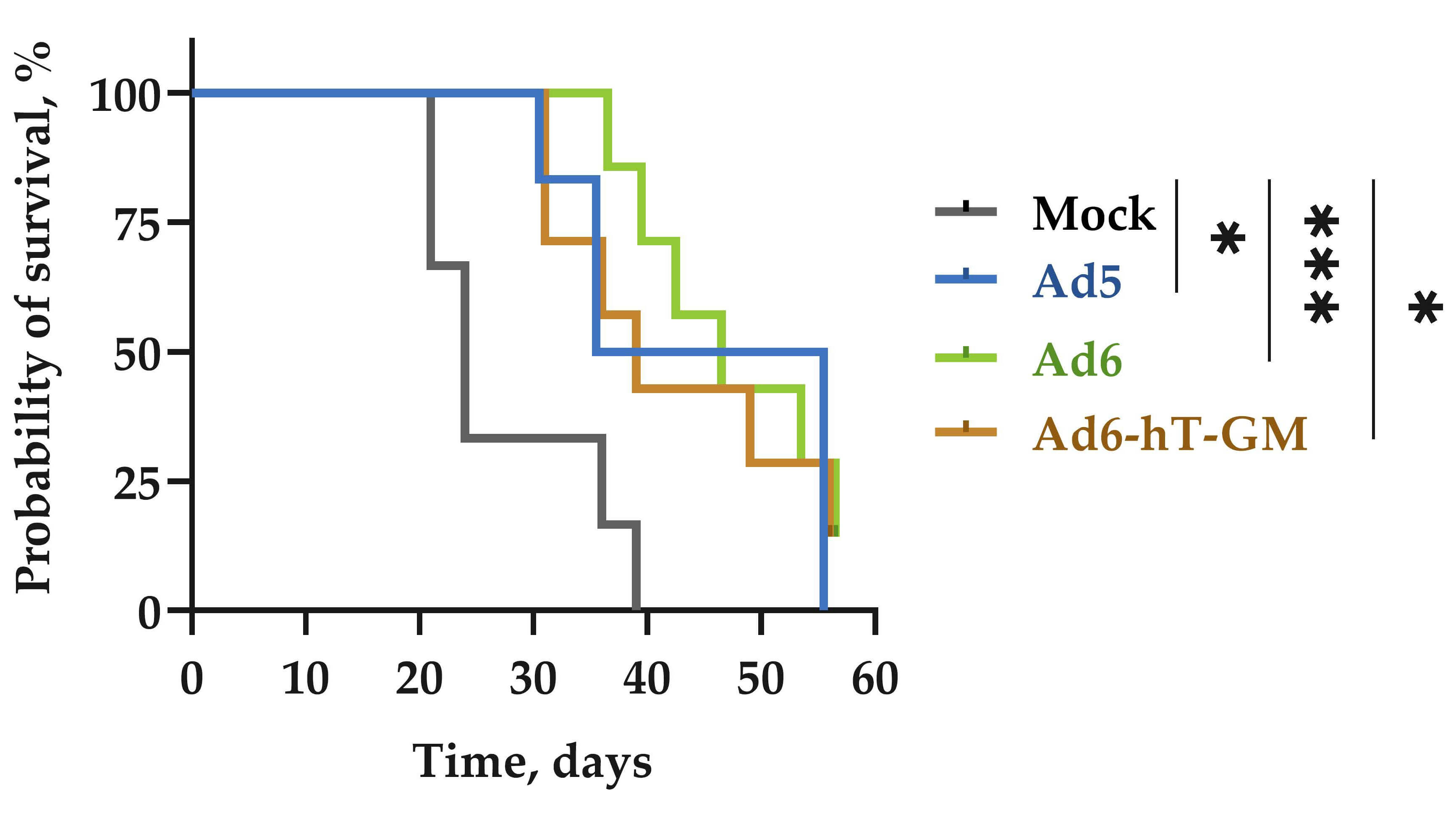 Fig.6 Adenovirus carrying hTERT promoter could prolong the survival time of tumor-bearing mice.2,4
Fig.6 Adenovirus carrying hTERT promoter could prolong the survival time of tumor-bearing mice.2,4
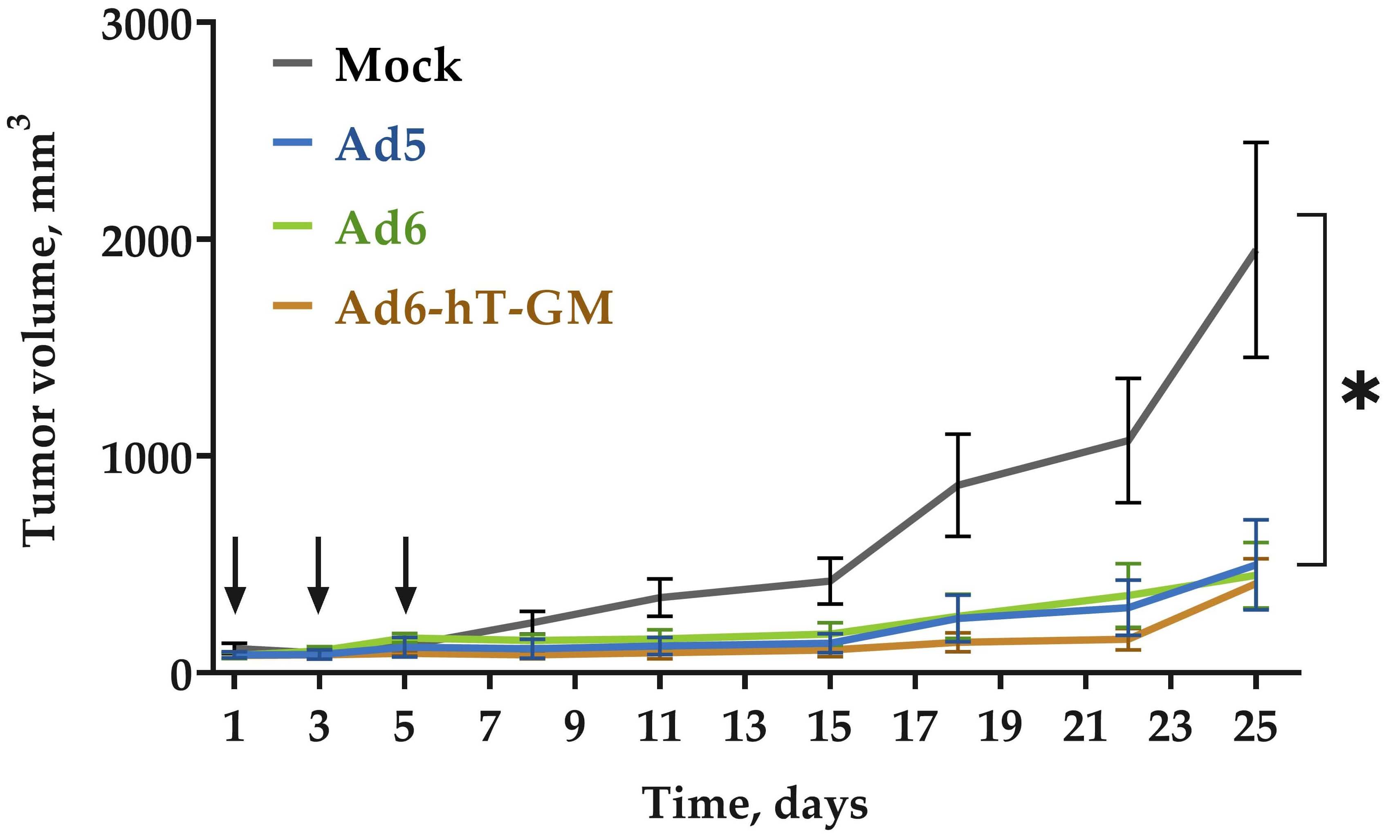 Fig.7 Adenovirus loaded with the hTERT promoter inhibited tumor growth.2,4
Fig.7 Adenovirus loaded with the hTERT promoter inhibited tumor growth.2,4