Cox2l Promoter driven Oncolytic Adenovirus Construction Service
Introduction
Our Cox2l Promoter-driven Oncolytic Adenovirus service addresses challenges such as cancer therapy resistance, limited advanced treatment options, and the need for specific anti-cancer agents. OncoVirapy™ Platform uses advanced genetic engineering and infectivity enhancement, we develop potent, tumor-selective virotherapies that deliver highly specific cancer cell killing, minimize off-target toxicity, and overcome resistance in hard-to-treat cancers.
[Discover How We Can Help - Request a Consultation]
Cox2l Promoter-driven Oncolytic Adenovirus
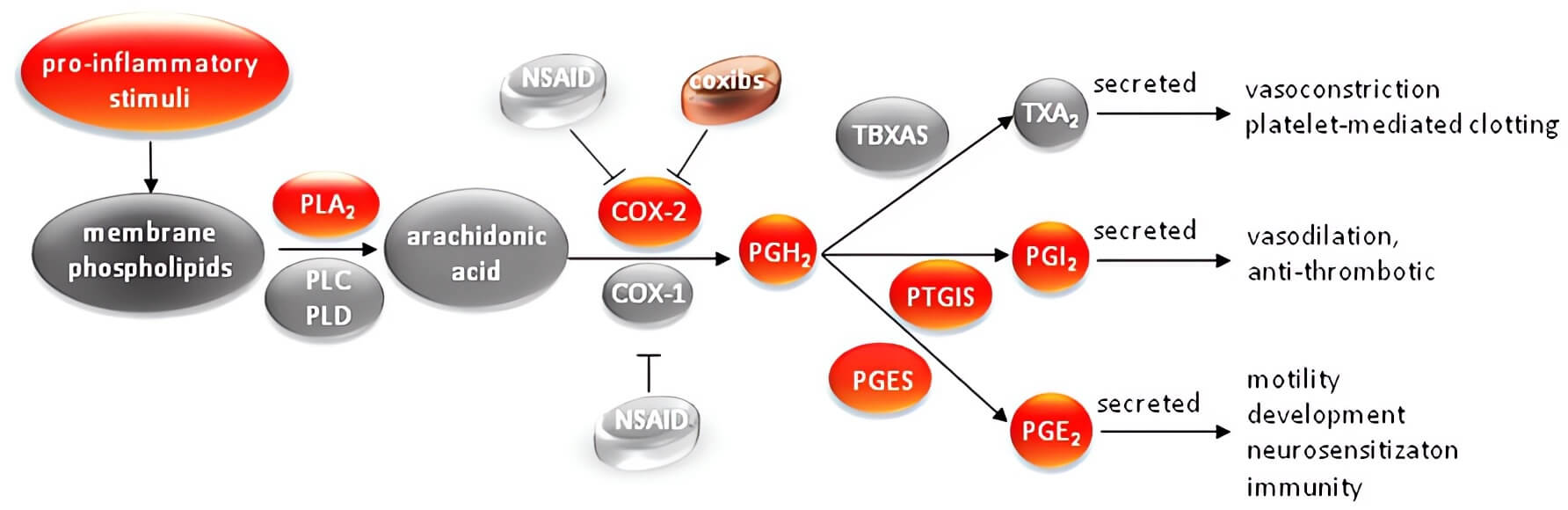 Fig.1 Pathways of action of COX2 in the inflammatory environment.1
Fig.1 Pathways of action of COX2 in the inflammatory environment.1
Cyclooxygenase-2 (COX-2) is an enzyme significantly overexpressed in a wide array of human cancers, including ovarian, prostate, pancreatic, and breast carcinomas while remaining at low or undetectable levels in most normal tissues. Its elevated activity during carcinogenesis makes it an ideal candidate for transcriptional targeting in gene therapy, allowing for selective gene expression within tumor cells.
Principle
The principle behind Cox2l Promoter-driven Oncolytic Adenoviruses lies in their tumor-specific replication, achieved through a sophisticated "triple-targeting" strategy:
- Transcriptional Control (Cox2l Promoter)
The Cox2l promoter regulates adenoviral E1A expression. In healthy cells with low COX-2, the promoter remains inactive, blocking viral replication. In COX-2-overexpressing cancer cells, it drives E1A expression for viral replication and oncolysis. As a "liver-off" promoter, it minimizes systemic toxicity.
- Trans complementary Targeting
E1A mutations prevent E1A-Rb binding. Most cancer cells with dysfunctional Rb pathways complement this defect to enable viral replication, while normal cells with intact Rb pathways cannot. Combining Cox2l promoter with E1A mutations enhances tumor specificity without reducing potency.
- Transduction Targeting (Serotype Chimerism)
Tumor cells often have low CAR receptor expression. Adenovirus capsid modification uses alternative receptors on cancer cells to improve viral entry and oncolysis efficiency.
Advantages
- High Tumor Specificity: Cox2l ensures precise replication primarily within COX-2 overexpressing tumor cells, greatly reducing replication and toxicity in normal tissues, particularly the liver.
- Potent Oncolytic Activity: Efficient viral replication within cancer cells leads to robust tumor cell lysis, which can be amplified through the spread of progeny virions to adjacent tumor cells.
- Enhanced Therapeutic Window: The multi-targeted design drastically improves the ratio of tumor-killing efficacy to normal tissue side effects, potentially allowing for higher, more effective therapeutic doses.
- Broad Applicability: This approach is relevant for a variety of solid tumors known to overexpress COX-2, including ovarian, prostate, pancreatic, and certain breast cancers, offering a versatile therapeutic platform.
- Overcoming Resistance: By targeting fundamental cancer-specific pathways and enhancing infectivity, these viruses can be effective against therapy-resistant tumor cells and difficult-to-treat phenotypes like neuroendocrine prostate cancer.
Workflow
| Required Starting Materials | Project Consultation & Design |
|---|---|
|
We start with an in-depth consultation to understand your research goals, target cancer type, and existing data. Our specialists then design a tailored oncolytic adenovirus strategy for optimal infectivity and therapeutic fit. |
| Vector Construction & Initial Validation | In Vitro Efficacy & Specificity Assessment |
| Our team genetically engineers your custom Cox2l promoter-driven oncolytic adenovirus, precisely cloning selected genetic elements into the adenovirus backbone. Post-construction, we conduct rigorous in vitro validation: viral titer determination (PFU/viral particles), replication kinetics, and Cox2l promoter activity confirmation in relevant cell lines. | We conduct in vitro studies via MTS/crystal violet assays on client cancer cell lines and normal cells to evaluate oncolytic potency and off-target toxicity. |
| Preclinical In Vivo Studies | Data Analysis & Comprehensive Reporting |
| For projects needing deeper validation, we design and conduct preclinical in vivo studies (subcutaneous xenograft/orthotopic models in immunocompromised mice). We monitor tumor growth, intratumoral viral replication, and animal survival to evaluate anti-tumor efficacy and systemic safety, providing critical translational data. | After all experimental phases, our team analyzes data with statistical evaluation. Clients will receive comprehensive reports including raw data, interpreted results, and mechanistic insights on your custom oncolytic adenovirus, supporting research and regulatory submissions. |
| Final Deliverables | Estimated Timeframe |
|
The typical timeframe for this service ranges from 8 to 14 weeks, depending on the complexity of the viral construct, the inclusion of optional preclinical evaluation, and the specific requirements of your project. More intricate designs or extensive in vivo studies may extend the duration. |
[Contact us to get more information]
What we can offer
Creative Biolabs is committed to empowering your oncology research with state-of-the-art Cox2l Promoter-driven Oncolytic Adenovirus solutions. We recognize that each project has unique requirements, and our offerings are designed to provide unparalleled flexibility and scientific rigor.
- Customized Oncolytic Adenovirus Design: Leverage our expertise to engineer bespoke Cox2l promoter-driven vectors. We select E1A mutations (e.g., Delta24) and serotype chimerism to match cancer targets and research goals, ensuring optimal specificity and efficacy.
- Comprehensive Preclinical Development Support: From vector construction and in vitro validation to in vivo efficacy studies in animal models, we provide a full development pipeline.
- High-Titer, Ultra-Purified Viral Production: Rely on our scalable production for high-titer, purified oncolytic adenoviruses. Stringent QC ensures consistency and reliability for experimental needs.
- Enhanced Tumor Specificity and Minimized Toxicity: Utilize our "triple-targeting" strategy to deliver vectors with expanded therapeutic windows and reduced off-target toxicity.
- Expert Scientific Consultation and Collaborative Partnership: Our specialists offer in-depth consultation, experimental design guidance, and data interpretation, acting as partners to maximize project success.
- Robust Quality Assurance and Documentation: Upholding high standards, we implement comprehensive quality systems, detailed documentation, and process analytics to ensure research integrity and reproducibility.
[Experience the Creative Biolabs Advantage - Get a Quote Today]
Case Study
| Oncolytic Virus Construction | Expression of COX2 |
|---|---|
|
|
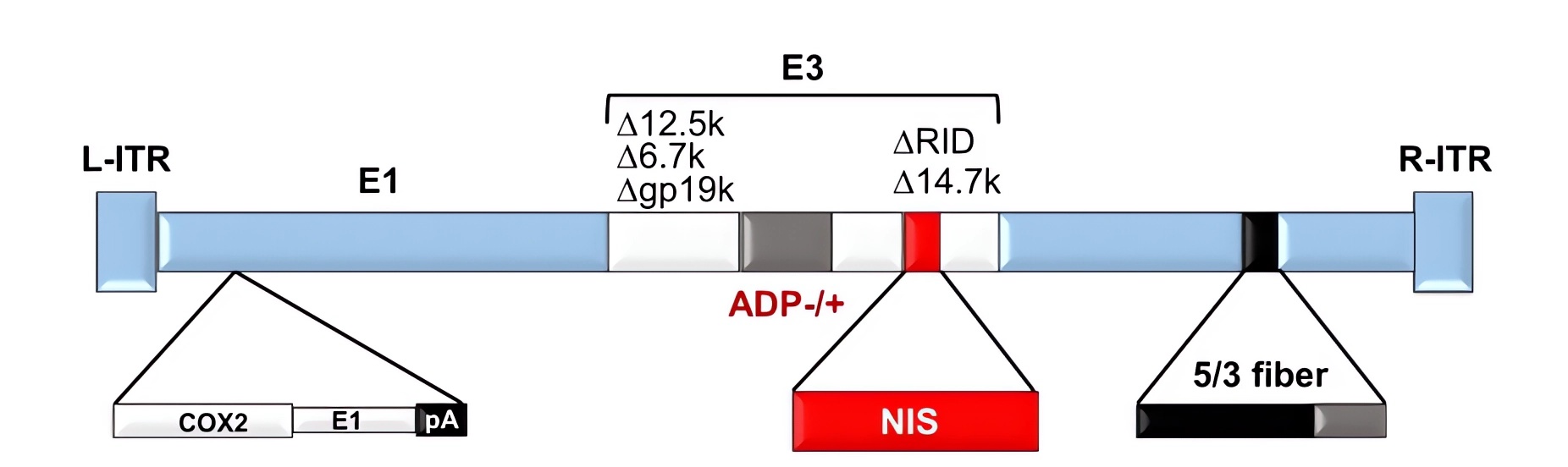
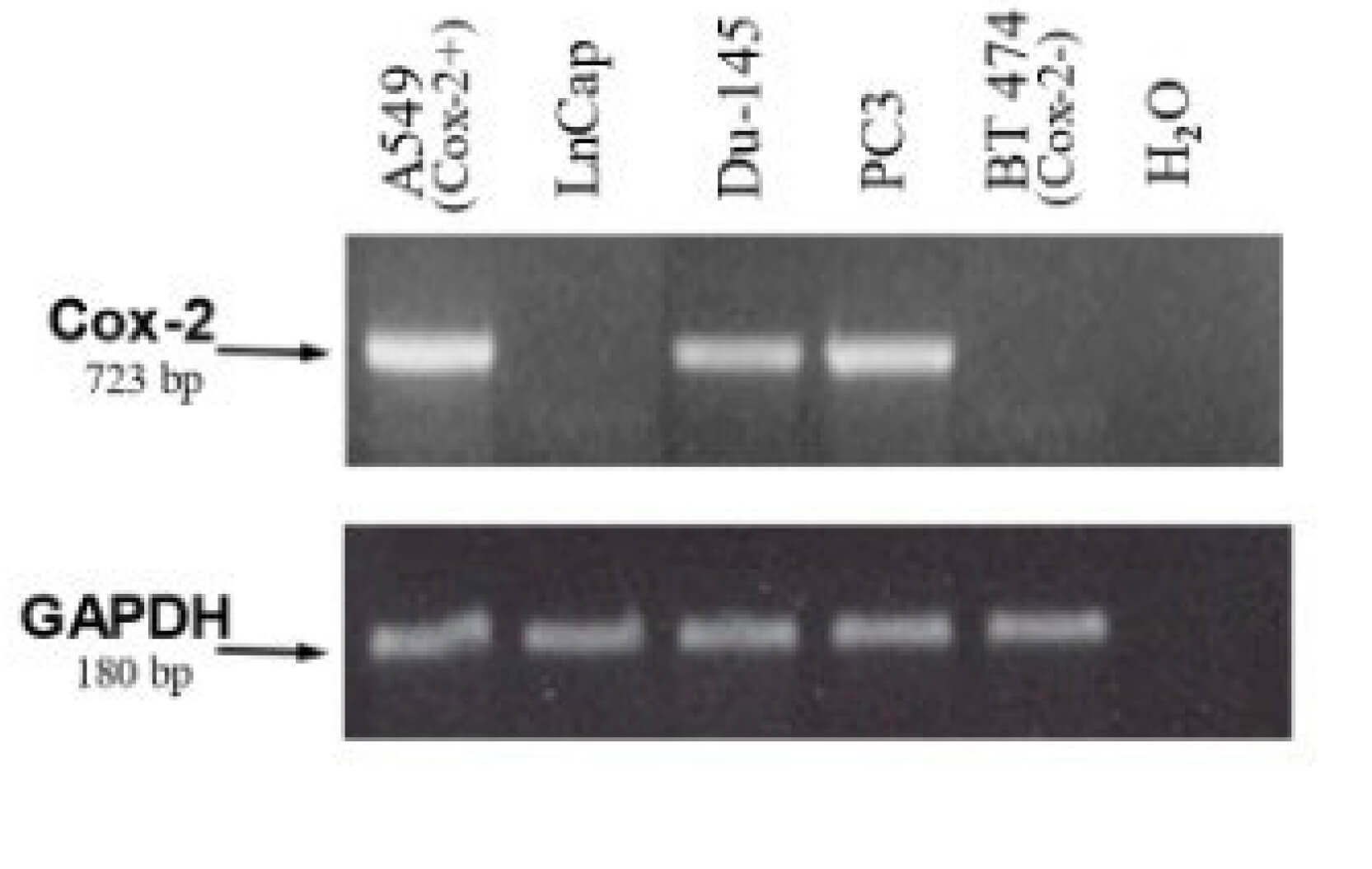
| Fig.2 The Cox2l promoter is inserted into the E1A region.2,4 | Fig.3 PCR primer amplification experiments were used to determine the RNA levels of COX.3,4 |
| Cytotoxicity | |
|
|
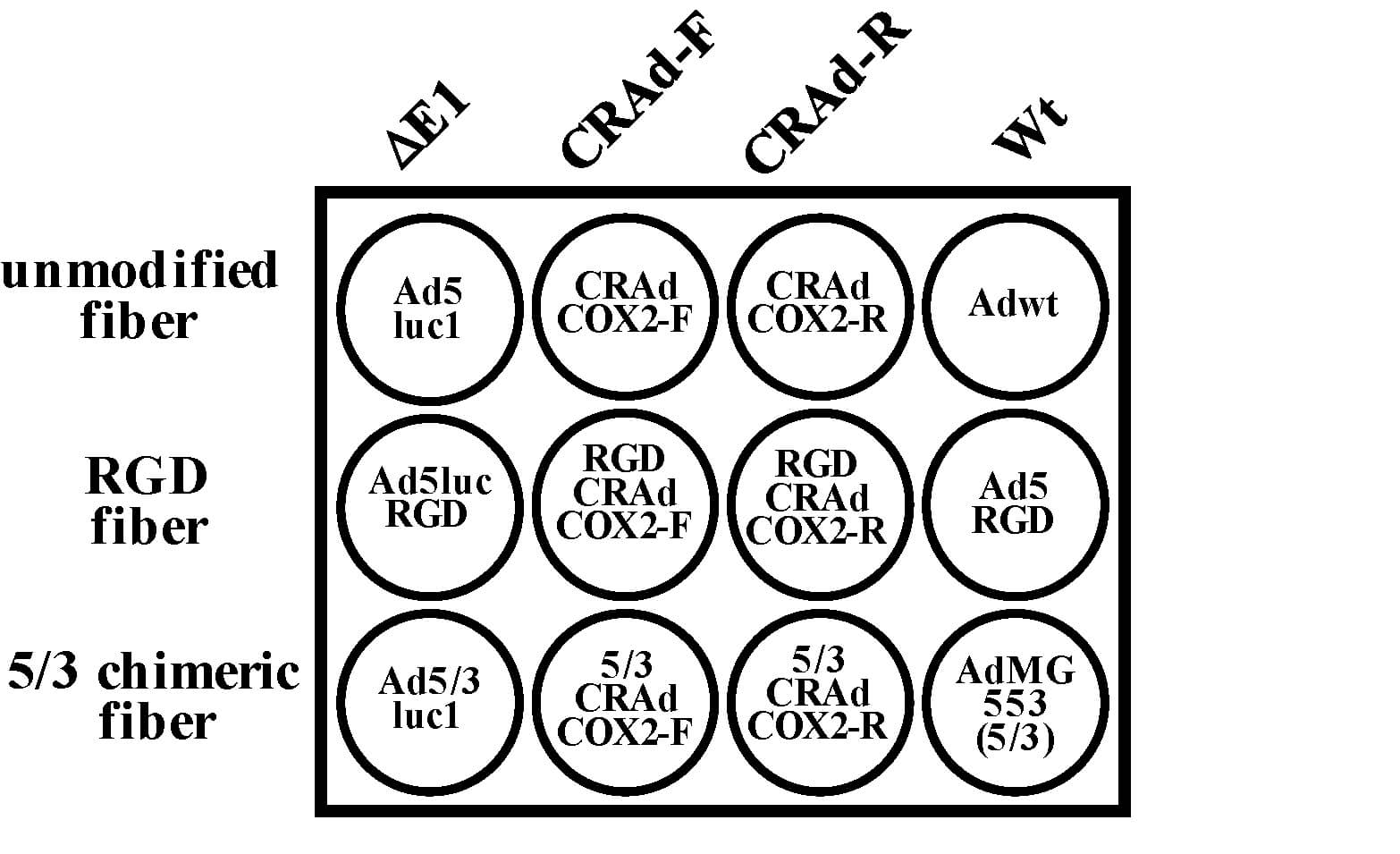
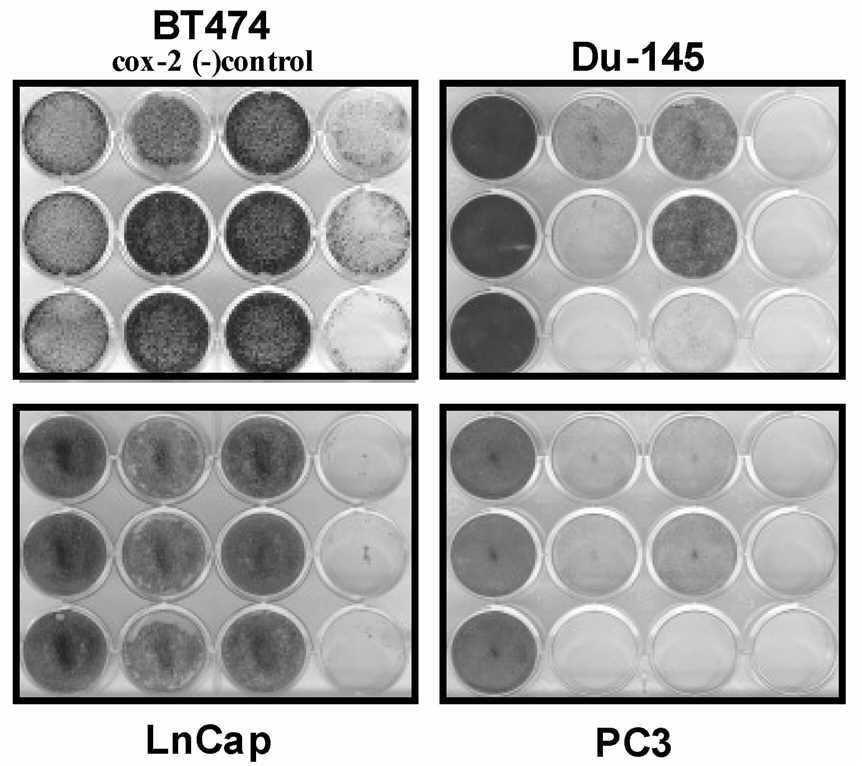
| Fig.4 Experimental design for detecting the cytotoxicity of oncolytic adenovirus to tumor cells.3,4 | Fig.5 The cytotoxicity of Cox2L-driven oncolytic adenovirus against tumor cells was examined using crystal violet staining.2,4 |
| Cell viability | Tumor Volume |
|
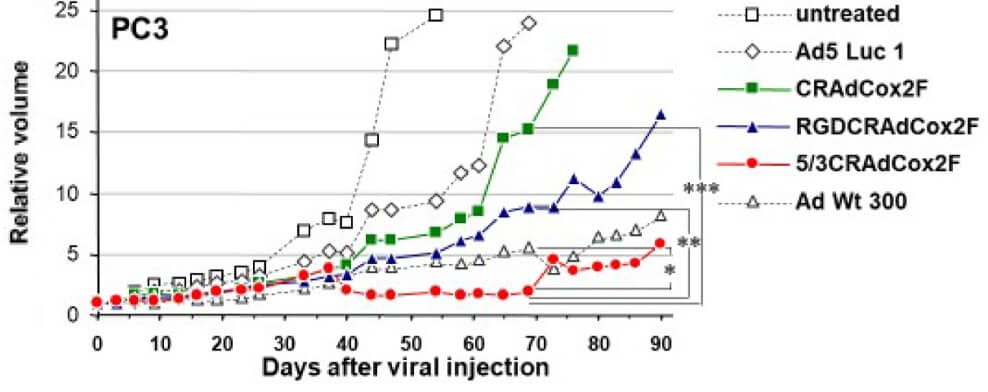
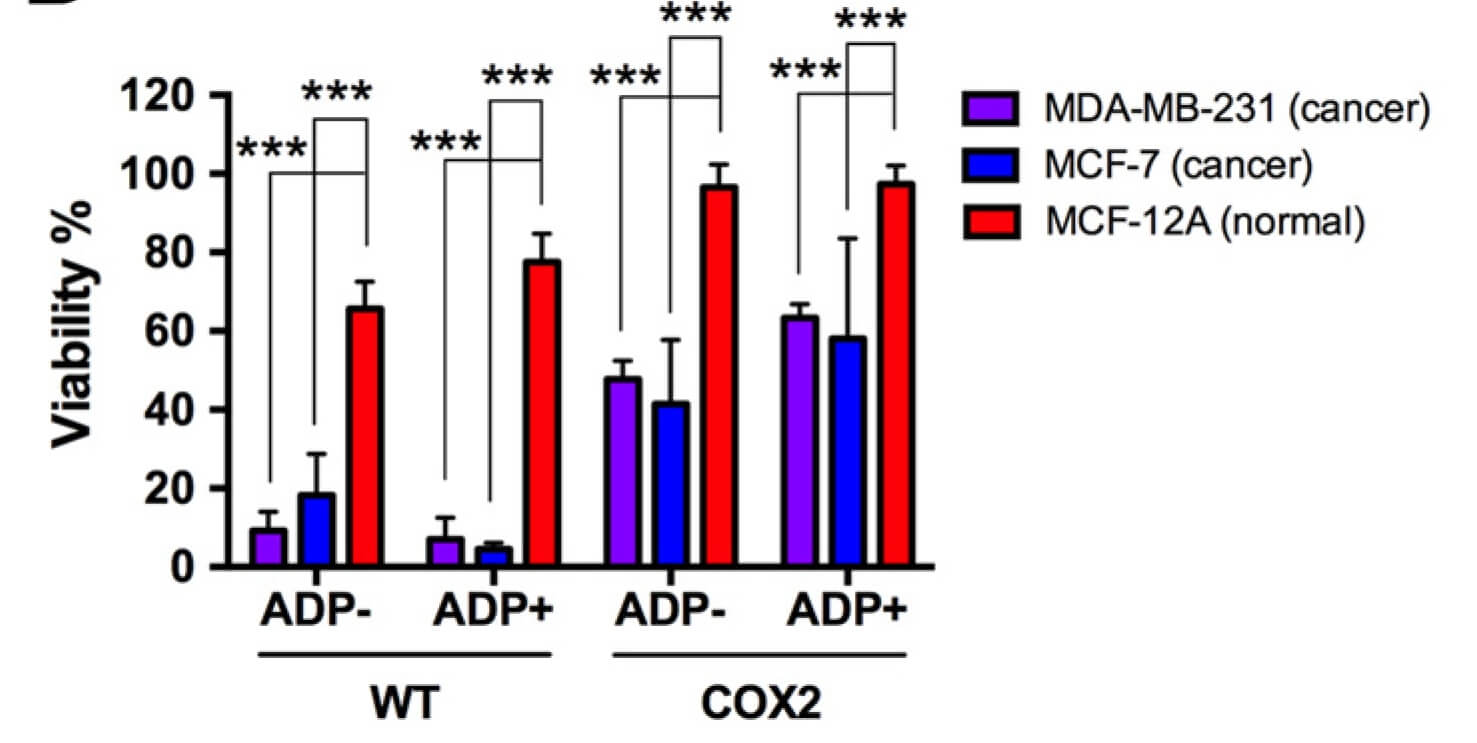
|
| Fig.6 The viability of tumor cells treated with oncolytic adenovirus is determined by crystal violet and counts as a bar graph.2,4 | Fig.7 Oncolytic adenovirus driven by Cox2l promoter can effectively slow tumor growth in mice.2,4 |
FAQs
What types of cancers are most suitable for Cox2l-driven oncolytic adenovirus therapy?
A: Our Cox2l-driven oncolytic adenoviruses target COX-2-overexpressing cancers like ovarian, CRPC, pancreatic, and some breast cancers. Consult us to assess your cancer model's suitability.
Can these oncolytic adenoviruses be combined with other cancer treatments?
A: Indeed, oncolytic adenoviruses are studied for combination therapies. Our Cox2l-driven vectors can flexibly integrate with chemo, radiation, immunotherapy, or small molecule inhibitors. Consult our team to optimize your combination strategy.
What are the key advantages of Creative Biolabs' Cox2l-driven approach compared to other oncolytic virus strategies or conventional therapies?
A: Our Cox2l-driven approach delivers key advantages: tight promoter control for superior tumor specificity, potent oncolysis in COX-2+ tumors, and enhanced infectivity in CAR-low cells via fiber modification. This design expands the therapeutic window and bypasses conventional treatment resistance.
What initial information does Creative Biolabs need to start a project involving Cox2l Promoter-driven Oncolytic Adenoviruses?
A: To start a project, we need your research objectives, target cancer type/cell lines, existing preclinical data, and desired outcomes. Providing the COX-2 expression status of target cells helps.
[Contact Our Team to Discuss Your Project]
Related Sections
References
- Stasinopoulos, Ioannis, et al. "COX-2 in cancer: Gordian knot or Achilles heel?." Frontiers in pharmacology 4 (2013): 34. DOI: 10.3389/fphar.2013.00034. Distributed under Open Access license CC BY 4.0, without modification.
- Robert, Sacha, et al. "Oncolytic Adenovirus for the targeting of Paclitaxel-resistant breast Cancer stem cells." Viruses 16.4 (2024): 567. DOI: 10.3390/v16040567
- Gavrikova, Tatyana, et al. "Infectivity-enhanced, conditionally replicative adenovirus for COX-2-expressing castration-resistant prostate cancer." Viruses 15.4 (2023): 901. DOI: 10.3390/v15040901
- Distributed under Open Access license CC BY 4.0, the figures were cropped.
