
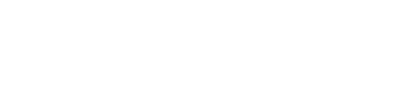
-
Solutions
- Subcellular Organelle Targeting Module Development
- Mitochondria Targeting Module Development
- Nucleus Targeting Module Development
- Cytosol Targeting Module Development
- Lysosome Targeting Module Development
- Golgi Targeting Module Development
Creative Biolabs enables precise drug delivery to subcellular organelles with our advanced targeting module platforms.
- Cell Targeting Module Development
- Neuron Targeting Module Development
- Microglia Targeting Module Development
- Astrocyte Targeting Module Development
- T Cell Targeting Module Development
- B Cell Targeting Module Development
- Macrophage Targeting Module Development
- NK Cell Targeting Module Development
- Neutrophil Targeting Module Development
- Endothelium Targeting Module Development
- Cancer Cell Targeting Module Development
Creative Biolabs develops cell-specific targeting modules to enhance therapeutic accuracy and efficacy.
- Microorganism Targeting Module Development
Creative Biolabs designs microorganism-targeting modules for precise antimicrobial and diagnostic applications.
- Disease Targeting Module Development
- Lymphoma Targeting Module Development
- Fibrosarcoma Targeting Module Development
- Glioma Targeting Module Development
- Hepatocellular Carcinoma Targeting Module Development
- Hepatitis Targeting Module Development
- Atherosclerosis Targeting Module Development
- Bladder Cancer Targeting Module Development
- Esophageal Cancer Targeting Module Development
- Alzheimer's Disease Targeting Module Development
- Hepatic Fibrosis Targeting Module Development
- Kidney Fibrosis Targeting Module Development
- Pulmonary Arterial Hypertension Targeting Module Development
- Osteosarcoma Targeting Module Development
- Lung Adenocarcinoma Targeting Module Development
- Diabetes Targeting Module Development
- Cervical Cancer Targeting Module Development
Creative Biolabs designs disease-specific targeting modules to improve drug delivery and therapeutic outcomes.
- Organ/Tissue Targeting Module Development
- Heart Targeting Module Development
- Liver Targeting Module Development
- Spleen Targeting Module Development
- Lung Targeting Module Development
- Kidney Targeting Module Development
- Bone Targeting Module Development
- Brain Targeting Module Development
- Prostate Targeting Module Development
- Adipose Tissue Targeting Module Development
- Eye Targeting Module Development
- Joint Targeting Module Development
Creative Biolabs creates organ- and tissue-targeting modules for site-specific drug delivery and enhanced efficacy.
- Subcellular Organelle Targeting Module Development
-
Services
- Module Delivery Systems
- Liposome
- LNP
- Exosome
- VLP
- Micelles
- Hydrogels
- Fc Fusion Protein
- Albumin
- CPP
- Graphene Oxide
- Hyaluronic Acid
- Elastin-like Polypeptide
- Polymeric Microsphere
- Electrospun Polymeric Nanofiber
Creative Biolabs provides tailored delivery system development to optimize the transport and release of therapeutic agents.
- Targeted Module & Chemical Synthesis
Creative Biolabs offers customized synthesis of targeting modules and chemical compounds for advanced drug delivery solutions.
- Bioconjugate
Creative Biolabs specializes in bioconjugation to link targeting modules with therapeutic payloads or delivery system for enhanced delivery.
- Characterization

Creative Biolabs specializes in the thorough characterization of targeted delivery systems, delivering complete insights to advance your research.
- Module Delivery Systems
- Products
-
Platform
- SORT LNP Platform
Creative Biolabs' SORT LNP platform enables precision organ targeting, developing next-generation lipid nanoparticles for highly specific therapeutic delivery.
- Drug Delivery Platform

For versatile small molecule delivery, Creative Biolabs offers a platform to engineer module delivery systems for targeted applications.
- In Vitro Assay Platform

Accelerate your research with Creative Biolabs' in vitro platform, which develops custom assays to characterize targeting and efficacy.
- In Vivo Assay Platform
Advance your therapeutic candidates with critical in vivo data from Creative Biolabs' PK/PD and biodistribution study platform.
- Manufacturing Platform

Creative Biolabs provides reliable, large-scale manufacturing of targeted delivery systems under strict quality control, ensuring consistency for your programs.
- Bioconjugation Platform
Bioconjugation is key to targeted delivery, and Creative Biolabs' platform provides expert services to create advanced conjugates.
- SORT LNP Platform
- Support & Resources
- Company


 Conventional Drug Delivery
Conventional Drug Delivery
 Targeted Drug Delivery
Targeted Drug Delivery
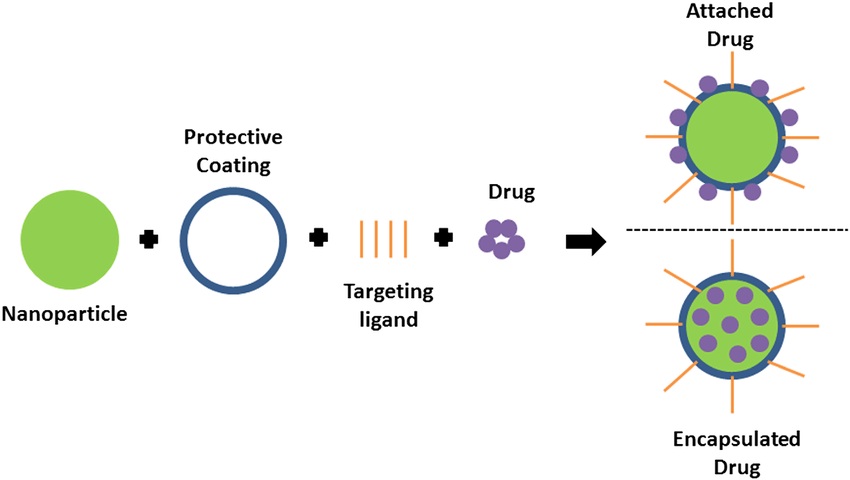 Fig.1 Scheme of drug loading options in targeted drug delivery.1
Fig.1 Scheme of drug loading options in targeted drug delivery.1 
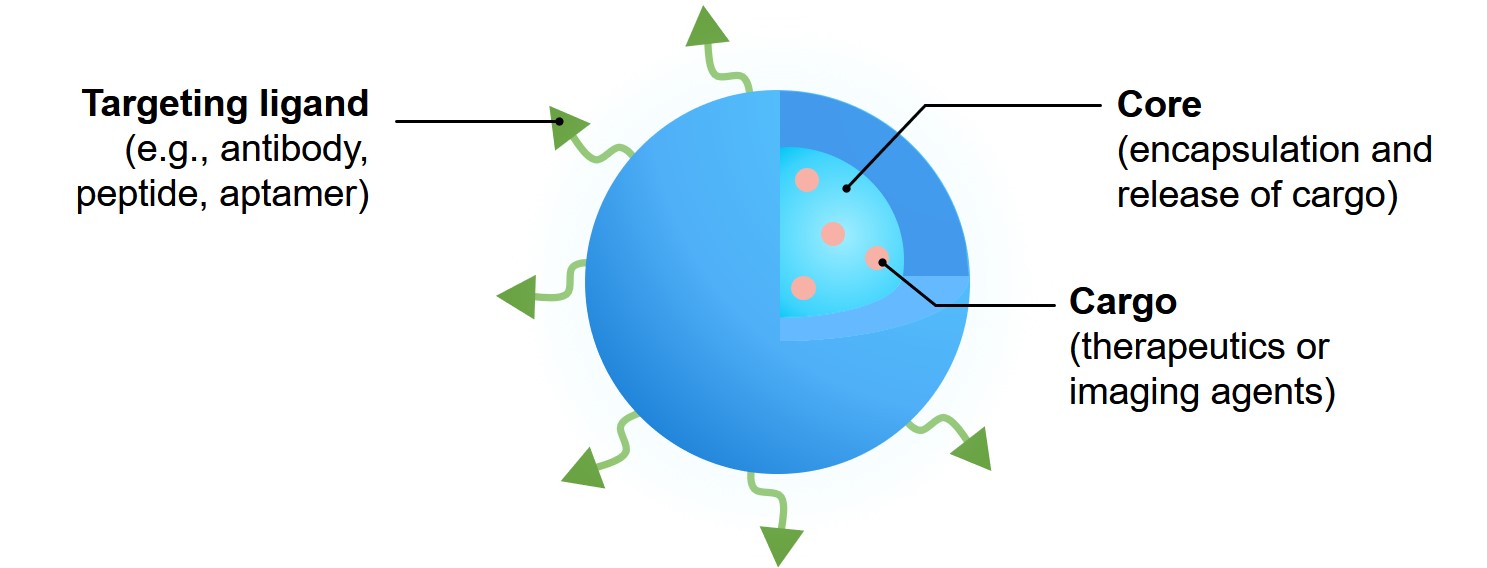 Fig 6. Nanoparticle platform for drug delivery.
Fig 6. Nanoparticle platform for drug delivery. 

