Highly potent cytotoxic drugs precisely delivered to cancer cells via monoclonal antibodies, minimizing systemic toxicity.
Drug-Ligand Conjugate Development Services
Are you currently facing challenges with non-specific drug delivery, off-target toxicity, or limited therapeutic windows in your biochemistry and biopharmaceutical projects? Creative Biolabs' Drug-Ligand Conjugates Services help you enhance drug specificity, reduce systemic side effects, and optimize therapeutic outcomes through precise molecular targeting.
Drug-Ligand Conjugates
Drug-ligand conjugates (DLCs) represent a transformative advancement in therapeutic delivery, offering a sophisticated strategy to enhance drug specificity and efficacy while minimizing systemic toxicity. At their core, DLCs are engineered molecules comprising a therapeutic agent (drug), a chemical linker, and a targeting ligand. The targeting ligand is a molecule specifically designed to recognize and bind to unique receptors or markers overexpressed on the surface of diseased cells, such as cancer cells or immune cells involved in inflammation. Upon binding, the conjugate is typically internalized into the target cell, where the drug is then released, often through the cleavage of the linker in response to specific intracellular conditions like pH changes or enzymatic activity.
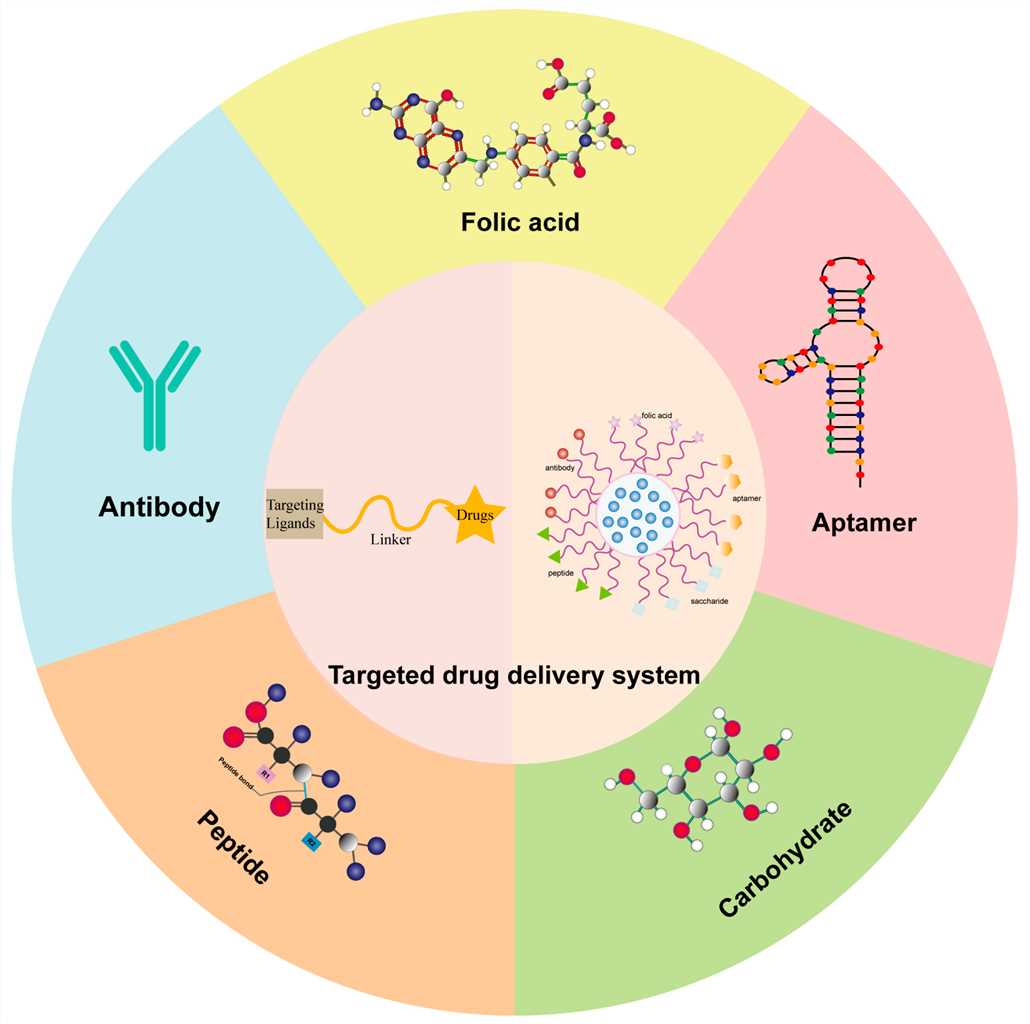 Fig.1 Different targeting ligands-mediated drug delivery systems.1,4
Fig.1 Different targeting ligands-mediated drug delivery systems.1,4
This targeted approach contrasts sharply with traditional systemic therapies, which often distribute drugs indiscriminately throughout the body, leading to undesirable side effects and limiting the maximum tolerated dose. The power of DLCs lies in their ability to concentrate the therapeutic payload precisely where it is needed, thereby maximizing therapeutic impact and safeguarding healthy tissues. For instance, research has explored various targeting ligands, including folic acid, carbohydrates, peptides, aptamers, and antibodies, to deliver drugs specifically to tumor cells, significantly enhancing therapeutic efficacy and reducing toxicity. Folic acid, for example, is widely utilized due to its overexpression in many cancer types and its favorable chemical properties, allowing for direct conjugation to small-molecule drugs or integration with nanomaterials. Furthermore, specific ligands like estrogen receptor (ER) ligands have been successfully incorporated into drug conjugates to target hormone-dependent cancers, offering a dual benefit of ER targeting and cytotoxic delivery. The development of melanotan-II (MT-II) conjugates for melanoma, which specifically target the MC1R receptor, further exemplifies this precision, demonstrating how self-cleavable linkers can facilitate drug release inside the acidic endosomal environment of cancer cells, thereby overcoming drug resistance. The ongoing advancements in DLC technology promise to unlock new possibilities for treating a wide array of diseases with unprecedented precision and improved patient outcomes.
Drug-Ligand Conjugates Services
Harness the latent capabilities of your therapeutic candidates through our dedicated Ligand Conjugation Solutions. We excel in designing, synthesizing, and characterizing innovative conjugates that enhance therapeutic efficacy, improve specificity, and reduce off-target effects.
Explore our diverse range of advanced conjugate platforms, each engineered for precise delivery and optimized performance:
Lipid-Drug Conjugates (LDCs)
Designed to improve drug solubility, enhance cellular uptake, and optimize bioavailability, particularly for challenging compounds.
Peptide-Drug Conjugates (PDCs)
Leveraging the inherent specificity and binding affinity of peptides to target disease-specific receptors or pathways.
Small Molecule-Drug Conjugates (SMDCs)
Utilizing the versatility of small molecules as targeting ligands for a broad spectrum of therapeutic applications.
Aptamer-Drug Conjugates (ApDCs)
Employing high-affinity RNA or DNA aptamers for precise targeting and delivery of therapeutic payloads.
Antibody-Oligonucleotide Conjugates (AOCs)
Combining the specificity of antibodies with the therapeutic potential of oligonucleotides for gene modulation or silencing.
Antibody Fragment-Drug Conjugates (FDCs)
Utilizing smaller antibody fragments for improved tissue penetration and reduced immunogenicity while maintaining targeting capabilities.
Radionuclide Drug Conjugates (RDCs)
Integrating radionuclides for highly sensitive diagnostic imaging and targeted radiotherapy in oncology.
Degrader-Antibody Conjugates (DACs)
DACs represent a nascent biotherapeutic category integrating antibody precision with catalytic protein elimination machinery.
Virus-Like Drug Conjugates (VDCs)
Harnessing the natural delivery mechanisms of virus-like particles for efficient and targeted payload delivery.
Immune-Stimulating Antibody Conjugates (ISACs)
Designed to deliver immune-activating agents directly to tumor cells or the tumor microenvironment, boosting anti-tumor immunity.
Ready to accelerate your drug development? Click below to discover how our expertise in drug-ligand conjugates can provide a precise and powerful solution for your next therapeutic breakthrough.
Contact Us About Bioconjugation Services
Application
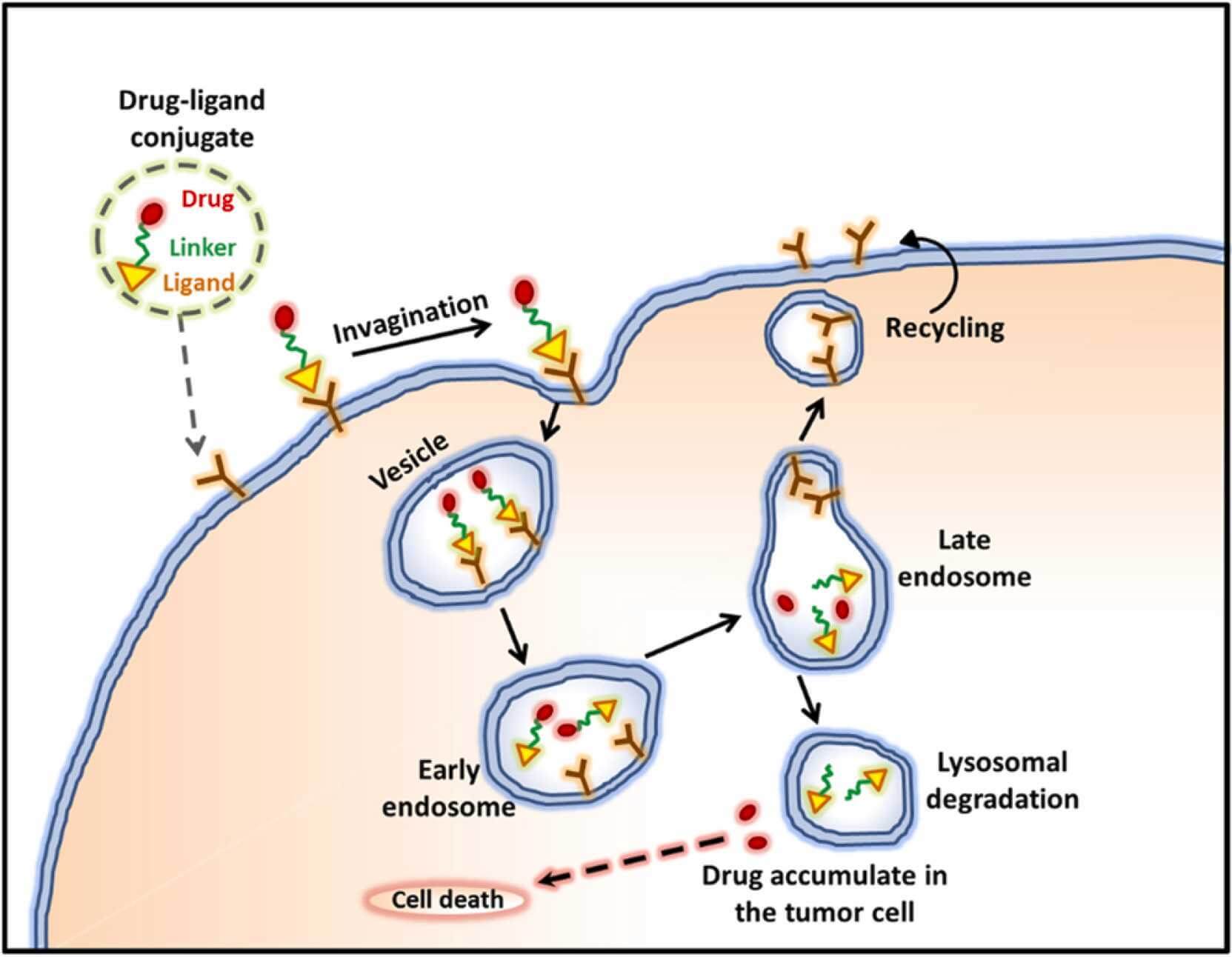 Fig.2 Receptor mediated ligand-drug conjugate endocytosis.2,4
Fig.2 Receptor mediated ligand-drug conjugate endocytosis.2,4
Drug-ligand conjugates and targeted delivery systems are transforming therapeutic interventions across oncology, immunology, and other fields.
- Targeted Cancer Therapy: DLCs precisely deliver cytotoxic agents to cancer cells, minimizing healthy tissue damage.
- Gene Therapy & Vaccines: Ligand-conjugated nanoparticles efficiently deliver genetic material for gene therapies and vaccine development.
- Autoimmune & Infectious Diseases: DLCs target specific cells, delivering drugs to inflammation sites and enhancing antimicrobial efficacy.
- Diagnostic Imaging: Ligand-conjugated probes enable precise molecular imaging.
- Overcoming Biological Barriers: DLCs can cross barriers like the blood-brain barrier for treating neurological disorders.
What We Can Offer?
Creative Biolabs is uniquely positioned at the forefront of targeted drug delivery innovation. Our team of expert biologists, chemists, and engineers brings over two decades of collective experience in developing sophisticated delivery solutions. We provide an extensive portfolio of solutions customized to address the varied requirements of your R&D initiatives:
Ready-to-Use Products
Access a comprehensive catalog of pre-formulated Module Delivery Systems, including advanced liposomes, exosomes, Lipid Nanoparticles (LNPs), and various polymeric nanoparticles. We also provide a selection of validated Targeted Modules such as high-affinity aptamers, specific peptides, functionalized lipids, targeted polymers, and responsive materials, all ready for immediate integration into your research and development workflows.
Customized Services
Our bespoke service allows us to develop tailored delivery systems and novel targeted modules from concept to validation. We precisely meet your project's unique specifications, including custom aptamer, peptide, or polymer synthesis and conjugation, as well as optimization of delivery system characteristics for specific cell subsets or disease contexts.
Conjugation Services
Leverage our extensive expertise in conjugating selected ligands to a wide array of delivery platforms, including various nanoparticles, liposomes, and polymers. Our meticulous conjugation techniques ensure stable and effective integration of your targeting moieties.
Pre-Clinical Validation
Benefit from our robust in vitro and in vivo testing capabilities to rigorously assess the targeting efficiency, cellular uptake, biodistribution, and therapeutic efficacy of your drug-ligand conjugates. This crucial step provides essential data for advancing your candidates.
Comprehensive Scientific Support
Partner with us to leverage our deep scientific knowledge, state-of-the-art facilities, and rigorous quality control for your targeted delivery projects. We provide end-to-end support, from experimental design consultation to in-depth data analysis, ensuring the success of your research.
Workflow
Why Choose Us?
Selecting Creative Biolabs facilitates accelerated pharmaceutical progression, enhanced clinical benefits, and significant reduction of adverse reactions. Our dedication to pioneering science guarantees your treatments precisely reach their destinations, unveiling fresh avenues for disease management.
Proven Expertise
Our highly specialized team of biologists, chemists, and engineers holds extensive scientific understanding and practical expertise in creating and evolving advanced drug delivery systems and targeting modules.
Innovative Technology
We utilize cutting-edge platforms for module synthesis, conjugation, and characterization, guaranteeing superior quality and the most innovative solutions for your specific projects.
Tailored Customization & Flexibility
We provide bespoke aptamer/peptide design and delivery system refinement, precisely aligning with your therapeutic objectives and target cell profiles. Our adaptable methodology adjusts to your distinct project demands.
Rigorous Quality & Reliability
Our steadfast dedication to scientific precision guarantees dependable, consistent, and excellent outcomes for your vital initiatives, instilling trust in your findings and progression.
Published Data
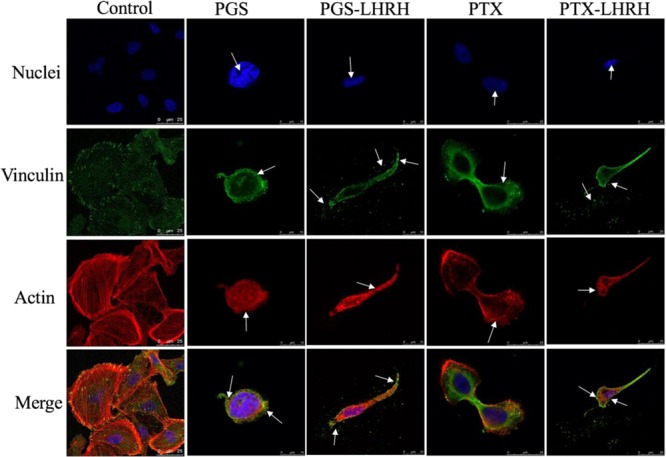 Fig.3 Images showing cellular uptake and cytotoxicity comparison of MDA-MB-231 cells 6 hours after their incubation with PGS, PGS-LHRH, PTX or PTX-LHRH.3,4
Fig.3 Images showing cellular uptake and cytotoxicity comparison of MDA-MB-231 cells 6 hours after their incubation with PGS, PGS-LHRH, PTX or PTX-LHRH.3,4
In a significant study demonstrating the efficacy of targeted drug delivery, researchers explored the use of Luteinizing Hormone-Releasing Hormone (LHRH)-conjugated drugs for the treatment of triple-negative breast cancer (TNBC). This research aimed to overcome the inherent limitations of traditional chemotherapy, such as its lack of specificity and associated toxic side effects. The methodology entailed covalently linking potent oncology agents (prodigiosin or paclitaxel) to LHRH, yielding LHRH-prodigiosin and LHRH-paclitaxel conjugates. These novel compounds were then rigorously tested on 4-week-old athymic female nude mice that had been induced with subcutaneous triple-negative xenograft breast tumors.
The study's primary objective was to determine if these LHRH-conjugated drugs could specifically target and effectively treat TNBC. The results were highly promising: injections of the LHRH-conjugated drugs successfully targeted, eliminated, or significantly shrank tumors across early, mid, and late stages of progression. Crucially, these therapeutic effects were observed without any apparent cytotoxicity to healthy tissues, as confirmed by both in vivo toxicity assessments and ex vivo histopathological examinations. The remarkable specificity and efficacy observed were attributed to the overexpression of LHRH receptors on breast cancer cells and tumors, which served as precise binding sites for the LHRH molecular recognition units integrated into the drug conjugates. Both in vitro and in vivo experiments consistently demonstrated that these conjugated drugs effectively inhibited the growth of breast cancer cells and tumors, highlighting the immense potential of ligand-conjugated drugs for highly specific and localized treatment of aggressive cancers like TNBC.
FAQs
Q: How do targeted conjugates enhance the effectiveness of therapies?
A: Targeted conjugates significantly enhance therapeutic effectiveness by precisely delivering the active agent directly to diseased cells or tissues. This minimizes exposure to healthy cells, leading to a higher concentration of the drug at the target site, increased potency, and a substantial reduction in systemic side effects. It means you can achieve better therapeutic outcomes with potentially lower overall doses.
Q: What types of targeting ligands can be incorporated into these conjugates?
A: A wide array of targeting ligands can be used, including antibodies, antibody fragments, peptides, aptamers, small molecules (like folic acid or hormones), and even carbohydrates. The choice of ligand depends on the specific target biomarker expressed on the diseased cells, ensuring high specificity and affinity for optimal delivery.
Q: Is this technology adaptable for different types of therapeutic agents?
A: Absolutely. This technology is highly versatile and can be adapted for various therapeutic agents, including small molecule drugs, nucleic acids (like siRNA or mRNA), proteins, and even imaging agents. The key is to design an appropriate linker and conjugation strategy that maintains the activity of both the therapeutic agent and the targeting ligand.
Q: What are the critical considerations when designing an effective drug-ligand conjugate?
A: Key considerations include the selection of a highly specific and potent targeting ligand, the choice of a suitable therapeutic payload, and the design of a cleavable or non-cleavable linker that ensures optimal drug release kinetics. Factors such as the stability of the conjugate in circulation, its pharmacokinetics, and its internalization mechanism into target cells are also crucial for successful design.
Q: How does this targeted approach compare to conventional systemic treatments?
A: Compared to conventional systemic treatments, the targeted approach offers several distinct advantages. It significantly reduces off-target toxicity, allowing for higher doses to be delivered specifically to the disease site. This can lead to improved therapeutic windows, enhanced patient safety, and potentially better treatment outcomes, especially for diseases where precise delivery is critical.
Creative Biolabs is committed to advancing the field of targeted drug delivery through our comprehensive Drug-Ligand Conjugates Services. Our expertise, innovative technologies, and dedication to quality empower your research to achieve unprecedented precision and efficacy in therapeutic development.
Connect with our experts for project-specific consultation and detailed insights.
References
- Yan, Shuxin et al. "Different Targeting Ligands-Mediated Drug Delivery Systems for Tumor Therapy." Pharmaceutics vol. 16,2 248. 7 Feb. 2024, DOI:10.3390/pharmaceutics16020248.
- Al-Mansoori, Layla et al. "Bio-vehicles of cytotoxic drugs for delivery to tumor specific targets for cancer precision therapy." Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie vol. 144 (2021): 112260. DOI:10.1016/j.biopha.2021.112260.
- Obayemi, J D et al. "LHRH-Conjugated Drugs as Targeted Therapeutic Agents for the Specific Targeting and Localized Treatment of Triple Negative Breast Cancer." Scientific reports vol. 10,1 8212. 19 May. 2020, DOI:10.1038/s41598-020-64979-1.
- Distributed under Open Access license CC BY 4.0, without modification.



