Years of specialized experience in targeted drug delivery and immunology, with a deep understanding of splenic biology and its role in disease.
Spleen Targeting Module Development Service
Accelerate Your Targeted Drug Delivery Research!
Are you currently facing challenges such as off-target toxicity, inefficient drug accumulation, or complex immunomodulation in your therapeutic development? Our Spleen Targeting Module Development services at Creative Biolabs help you enhance therapeutic efficacy, minimize systemic side effects, and optimize drug distribution through advanced module engineering, precision delivery platforms, and comprehensive in vitro and in vivo validation.
Contact our team to get an inquiry now!
Overview
The spleen, a pivotal lymphoid organ, orchestrates critical immune functions—systemic filtration of particulate matter, pathogens, and defective erythrocytes alongside immune response activation. Its unique anatomical architecture, featuring specialized sinusoidal vasculature and sluggish hemodynamics, creates optimal conditions for precision therapeutic delivery. Phage-derived homing peptides enable targeted payload navigation to specific splenic zones. Due to reciprocal hemodynamics between splenic and hepatic circulation, nanoparticle accumulation inversely correlates with hepatic uptake, necessitating nanovectors that evade Kupffer cell-mediated sequestration post-IV administration. Advances in organ-selective vectorization have yielded non-biological platforms—liposomal carriers, polymeric nanovehicles, solid lipid matrices, and inorganic nanostructures—engineered to maximize splenic retention through optimized biodistribution.
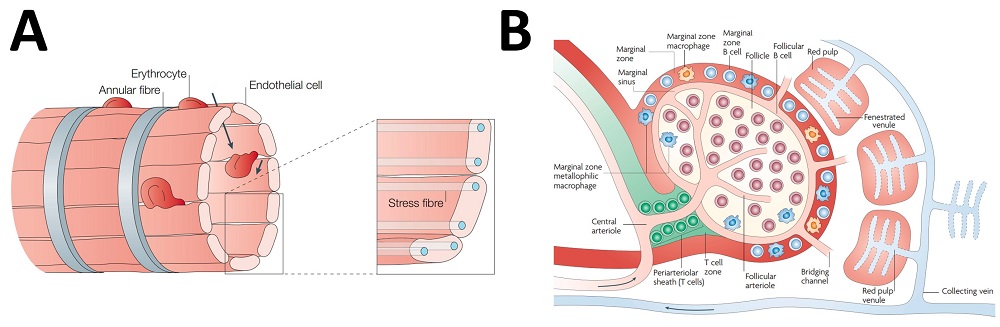 Fig.1 Microanatomy of the spleen circulation.1,3
Fig.1 Microanatomy of the spleen circulation.1,3
Delivery System Targeting Spleen
Splenic vectorization strategies offer dual clinical advantages through pathology-selective accumulation. Enhanced therapeutic bioavailability within splenic tissues amplifies treatment efficacy against infections localized to this organ and hematologic disorders including malaria and autoimmune hemolytic anemia. Simultaneously, radiolabeled imaging probes enable high-resolution visualization of splenic architecture, facilitating accurate detection of pathological alterations for diagnostic evaluation.
The spleen's expanded vascular fenestrations and permissive hemodynamics enable entrapment of macromolecular particulates, distinguishing it from hepatic filtration pathways. This underscores splenic vectorization merits prioritization in therapeutic design, necessitating mechanistic insights to optimize splenotropic delivery mechanisms. A critical hurdle involves engineering nanovectors to circumvent hepatic sequestration while enhancing splenotropic biodistribution. Key determinants—nanocarrier biophysical parameters, lipidomic profiles, and corona adsorption dynamics—require systematic optimization to modulate hepatosplenic trafficking.
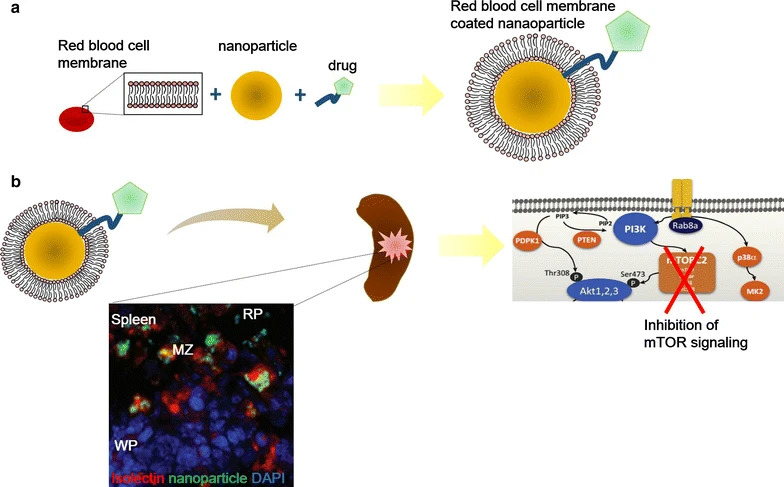 Fig 2. The potential usage of nanoparticles for splenic targeting.2,3
Fig 2. The potential usage of nanoparticles for splenic targeting.2,3
Splenic vectorization strategies bifurcate into biophysical optimization and molecular recognition paradigms. Passive approaches leverage nanocarrier dimensions (e.g., hydrodynamic diameter, ζ-potential), structural conformation, and rigidity to modulate biodistribution. PEGylation prolongs systemic residence by evading immune clearance, while pH-actuated nanovectors exploit macrophage lysosomal acidity for stimuli-responsive payload release. Active methodologies employ bioaffinity molecules—antibody-derived constructs, carbohydrate motifs (mannose/sialic acid analogs), or oligonucleotide-based probes—to engage splenic cellular receptors. Lipid-based nanocarriers (LNPs) further demonstrate composition-dependent tropism, where phospholipid profiling directs payload partitioning to discrete splenocyte subsets. Compositional fine-tuning of LNPs proves critical for advancing nucleic acid therapeutics, particularly mRNA vaccine platforms requiring precise immune cell targeting.
What We can Offer?
Creative Biolabs is your comprehensive partner for Spleen Targeting Module Development, offering a robust suite of products and services designed to meet diverse research and therapeutic needs.
- Custom Spleen-Targeting Ligand Discovery & Design
- Targeting Module Synthesis & Conjugation
- Carrier System Optimization for Spleen Delivery
- Comprehensive In vitro Validation
- Advanced In vivo Biodistribution & Efficacy Studies
- Immunogenicity Assessment
- Analytical & Characterization Services
Experience the Creative Biolabs Advantage - Get a Quote Today
Why Choose Us?
Unrivaled Expertise
Proprietary Platform
Access to our advanced module engineering and conjugation platforms, enabling the rapid design and synthesis of highly specific targeting moieties.
Comprehensive Validation
Rigorous in vitro and in vivo validation capabilities, including advanced imaging and functional assays, to confirm splenic specificity and therapeutic efficacy.
Customized Solutions
Flexible and tailored approaches to meet the unique requirements of each project, from novel ligand discovery to carrier optimization.
Accelerated Development
Our streamlined workflow and integrated services significantly reduce development timelines, bringing your targeted therapeutics to fruition faster.
Published Data
Our methodologies and success stories are supported by extensive internal research and published data, demonstrating our commitment to scientific rigor and innovation.
Workflow
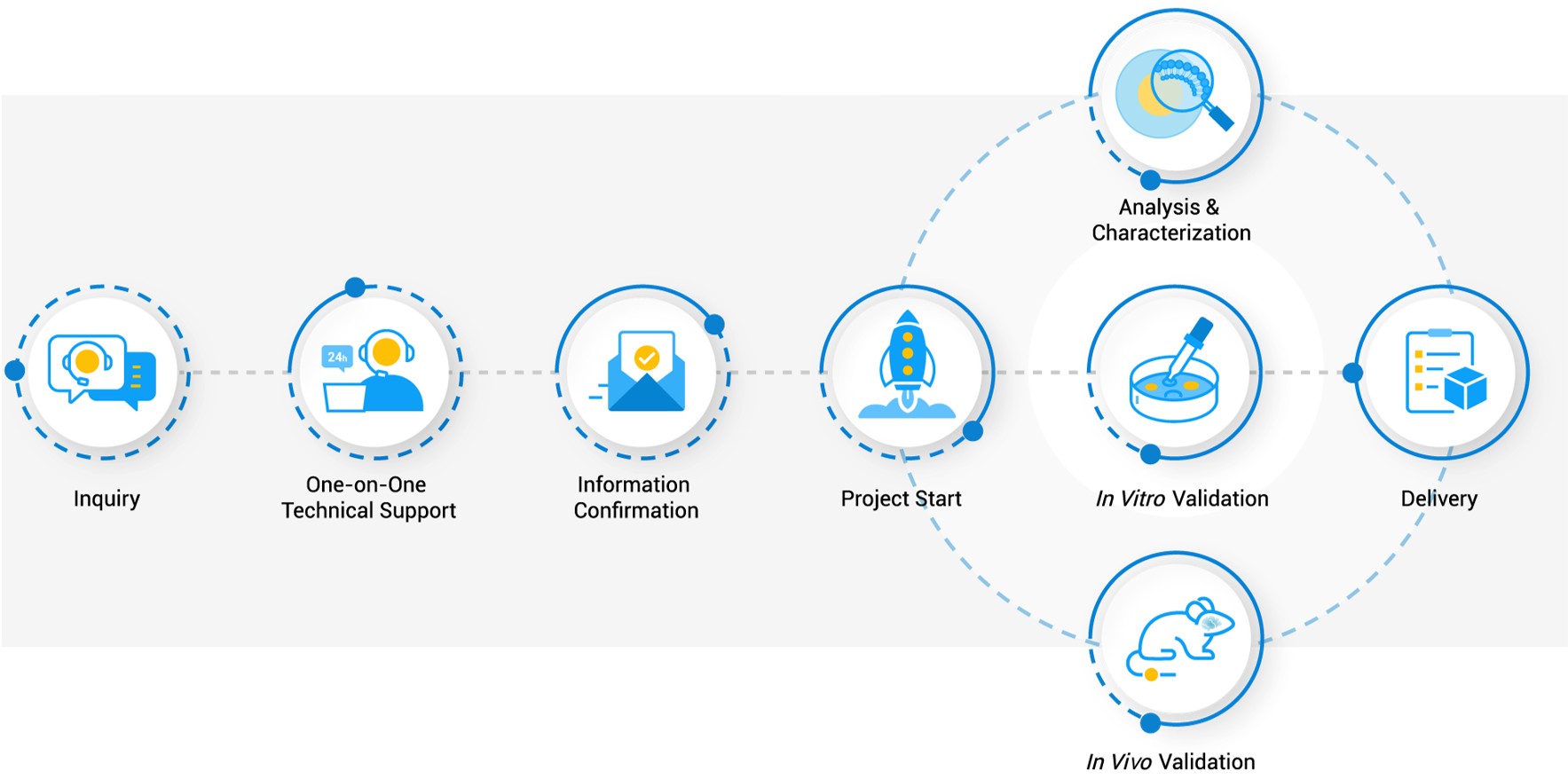
FAQs
Here are some common questions we receive about Heart Targeting Module Development:
What methodologies ensure the specificity of Creative Biolabs' splenic-targeting platforms?
Our integrated methodology initiates with precision ligand engineering against cellular epitopes unique to splenocytes. Subsequent validation involves ex vivo screening assays with native splenocyte populations and systemic biodistribution analyses via multimodal imaging to validate tropism toward splenic compartments while mitigating ectopic deposition.
What cargo types are compatible with Creative Biolabs' splenic vectorization platforms?
Our modular architecture supports diverse therapeutic agents—low-molecular-weight compounds, macromolecular biologics (antibodies/enzymes), nucleic acid payloads (siRNA/mRNA/gene therapy plasmids), and vaccine antigens. Payload-specific optimization ensures precise engineering of vector architecture and delivery mechanisms to maximize target tissue bioavailability.
Does Creative Biolabs support preclinical efficacy validation for splenic-targeted therapeutics?
Our capabilities span full-cycle development, including preclinical validation in pathologically relevant models. We quantify pharmacodynamic responses, therapeutic indices, and toxicological parameters to holistically evaluate target engagement and safety margins. Connect with our translational science team to design a customized validation cascade for your therapeutic candidate.
Creative Biolabs provide tailored targeted delivery solutions addressing unique research and therapeutic requirements. To explore these capabilities, please contact us for more information.
References
- Cataldi, Mauro, et al. "Emerging role of the spleen in the pharmacokinetics of monoclonal antibodies, nanoparticles and exosomes." International journal of molecular sciences 18.6 (2017): 1249. doi:10.3390/ijms18061249
- Li, Liang, et al. "The spleen in liver cirrhosis: revisiting an old enemy with novel targets." Journal of translational medicine 15 (2017): 1-10. doi:10.1186/s12967-017-1214-8
- Distributed under Open Access license CC BY 4.0, without modification.



