Golgi Targeting Module Development Service
Accelerate Your Targeted Drug Delivery Research!
Are you currently facing challenges in delivering your therapeutic payloads precisely to intracellular targets? Our Creative Biolabs' Golgi Targeting Module Development service helps you achieve efficient and specific drug delivery by leveraging advanced targeting module technology. We enable you to overcome the limitations of conventional drug delivery methods and enhance the efficacy of your therapies through innovative Golgi-targeted strategies.
Contact our team to get an inquiry now!
Overview
Functioning as the central hub of vesicular transport, the Golgi apparatus maintains dynamic interplay with the endoplasmic reticulum (ER). This organelle's core processing site enables vital post-synthesis biochemical alterations—phosphorylation, acylation, glycosylation, methylation, and sulfation—mediated through specialized enzymatic systems. M6P tagging serves as a principal molecular address that directs lysosomal sorting of mature biomolecules. Genetic anomalies, pharmacological interventions, or overexpression of Golgi-resident proteins induce structural perturbations in this compartment. Notably, structural abnormalities within neuronal Golgi networks underlie pathologies including Alzheimer's, Parkinson's, and Niemann-Pick diseases. Therapeutically, Golgi machinery is emerging as strategic intervention points in oncology. Golgi-targeted compounds demonstrate efficacy across androgen-responsive and castration-resistant prostate malignancies.
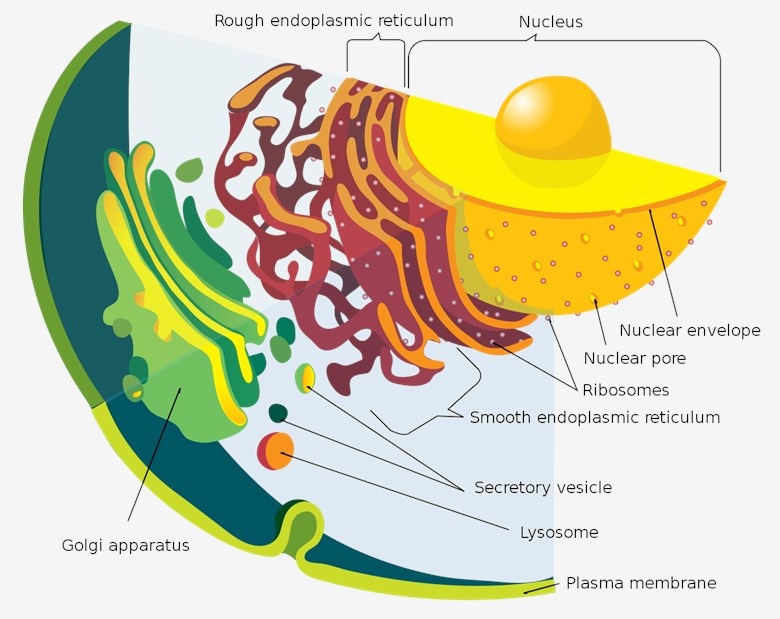 Fig.1 The endomembrane system.1,3
Fig.1 The endomembrane system.1,3
Golgi Targeting Strategy
The ER-Golgi intracellular transport axis represents an emerging therapeutic focus in oncology, with diverse pharmacological approaches demonstrating efficacy in directing molecular cargo to this secretory pathway nexus.
- Golgi Apparatus Trafficking Disrupting Agents
Functioning as the biosynthetic nexus of vesicular transport, the Golgi apparatus coordinates biomolecule maturation, modification, and directional trafficking through the secretory continuum. This pathway's dysregulation—particularly in oncogenic vesicular flux—drives malignant transformation through aberrant cargo secretion. Pharmacologically modulating these pathological transport mechanisms offers promising avenues for molecular oncology interventions. Brefeldin A, a fungal-derived macrocyclic lactone, represents a prototypical Golgi-distrupting agent that inhibits ARF1 GTPase/GEF complex formation. Such disruption induces GA-ER membrane coalescence and endosomal tubulogenesis, conferring cytotoxic and apoptosis-activating properties across diverse neoplastic cellular systems.
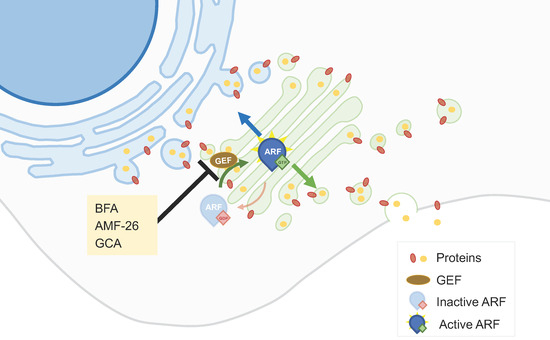 Fig 2. ARF1 GEF interaction inhibitors.2,3
Fig 2. ARF1 GEF interaction inhibitors.2,3
- Golgi-Milieu-Disrupting Agents
Monensin, a Streptomyces cinnamonensis-derived polyether ionophore, is characterized as a Golgi-targeting agent through its membrane-integrated cationophoric activity. The compound disrupts ionic gradients, precipitating rapid Golgi distension and aberrant vesicular transport. Furthermore, it modifies the organelle's luminal pH, compromising post-translational modification cascades. Such Golgi architectural perturbations initiate organelle-specific stress signaling cascades culminating in apoptotic elimination. Notably, this antibiotic demonstrates selective cytotoxicity toward neoplastic cells exhibiting mesenchymal transition phenotypes.
- Inhibitors of the Synthetic Glycosilation Machinery
Suppressing glycosidase activity disrupts glycan synthesis on membrane-bound glycoconjugates, a mechanism widely explored in oncology research. These agents alter malignant cell glycosylation patterns, promoting truncated O-glycan display and amplifying Tn antigen-antibody interactions, thereby strengthening immune-targeted tumor eradication through enhanced therapeutic synergy.
What We can Offer?
Creative Biolabs has a complete module delivery system and an experienced team of scientists. We offer:
- Individual targeting modules.
- Different types of module-payload/carrier complexes for specific subcellular organelles.
- A wide range of corresponding products.
- In vitro and in vivo validation of targeting module efficacy and specificity.
- Consultation and support throughout the project lifecycle.
Experience the Creative Biolabs Advantage - Get a Quote Today
Why Choose Us?
Creative Biolabs is a leading provider of advanced targeted delivery solutions, with extensive expertise in Golgi targeting module development. We leverage cutting-edge technologies and a team of experienced scientists to deliver solutions that meet your specific research and development needs.
- Expertise: Creative Biolabs has a team of experienced scientists with extensive knowledge in Golgi biology, drug delivery, and bioconjugation chemistry.
- Customization: We offer tailored solutions to meet your specific research needs, from designing novel targeting modules to developing customized conjugates.
- Advanced Technology: Creative Biolabs utilizes state-of-the-art technologies and platforms to ensure the highest quality and efficiency in our services.
- Published Data: Our work is supported by strong scientific evidence and Published Data, demonstrating the efficacy and reliability of our Golgi-targeting solutions.
Workflow

FAQs
How does Golgi targeting enhance drug delivery?
Subcellular targeting strategies focused on the Golgi apparatus optimize pharmaceutical delivery by concentrating therapeutic payloads within this critical organelle. Such precision enhances localized agent bioavailability while minimizing systemic toxicity through selective intracellular compartmentalization.
What types of therapeutic payloads can be targeted to the Golgi?
Multiple bioactive compounds—including small-molecule pharmaceuticals, peptide constructs, biologics, and genetic therapeutics—can be selectively routed to Golgi compartments. Our adaptive targeting scaffold system allows precision engineering of delivery architectures tailored to unique molecular cargo profiles.
What strategic benefits differentiate Golgi-precision pharmacology from conventional delivery paradigms?
Subcellular targeting to the Golgi enhances therapeutic precision through localized drug activation, circumvents systemic biodistribution errors, and disrupts resistance-linked cellular adaptation. Spatial confinement of therapeutics within this secretory organelle amplifies site-specific pharmacodynamics while negating collateral tissue damage.
What considerations apply to Golgi-directed therapeutic agents?
Although Golgi-centric pharmacological strategies demonstrate clinical promise, careful evaluation of nonspecific biodistribution and vector precision parameters remains crucial. Our research collective employs multi-stage optimization protocols, including structure-function modeling and in silico validation, to engineer Golgi-centric delivery architectures that mitigate these concerns.
What criteria determine ideal Golgi-directed vector selection?
Optimal vector identification involves assessing three core parameters: target biological system, cargo physicochemical profile, and required precision thresholds. Creative Biolabs' molecular engineering team provides precision-guided vector customization to align delivery platforms with experimental objectives.
Creative Biolabs maintains the life science sector's most comprehensive service array for lysosome targeting solutions. Please contact us for more information.
References
- Khine, Myat Nyein, and Kaori Sakurai. "Golgi-Targeting Anticancer Natural Products." Cancers 15.7 (2023): 2086.
- Martins, Marta, et al. "The golgi apparatus as an anticancer therapeutic target." Biology 13.1 (2023): 1.
- Distributed under Open Access license CC BY 4.0, without modification.



