Virus-Like Particle (VLP) Development Service
The critical bottleneck in modern therapeutics is targeted delivery. To truly maximize efficacy and minimize systemic toxicity, a delivery vehicle must navigate biological barriers and localize with pinpoint accuracy. Creative Biolabs' virus-like particle (VLP) development services transform these naturally occurring, non-infectious protein cages into advanced, customizable drug carriers. We engineer VLPs to encapsulate diverse payloads—from mRNA to small molecules—with superior stability and control.
What Are Virus-Like Particles (VLPs)?
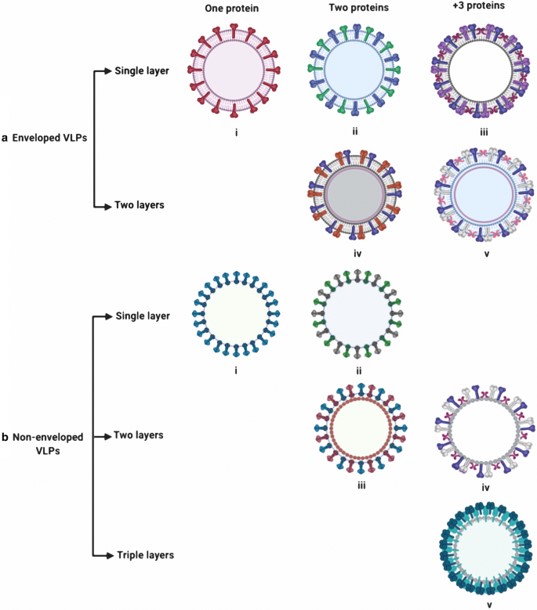 Fig. 1 Classification of various VLPs structure.1,3
Fig. 1 Classification of various VLPs structure.1,3
VLPs are multi-subunit protein structures that mimic the overall size, shape, and conformation of a native virus but are entirely non-infectious due to the intentional absence of the viral genome. They spontaneously assemble into highly stable, nanoscale structures, typically between 20 nm and 150 nm, presenting a repeating, high-density array of surface proteins. This intrinsic self-assembly and structural robustness make VLPs ideal, versatile scaffolds for high-density ligand display and protected internal cargo encapsulation, positioning them as a premier platform in modern nanomedicine.
Key Advantages of the VLPs:
- Enhanced Safety Profile: Non-infectious and safe, offering a superior profile over live viral vectors.
- Stability & Protection: Robust capsid shields sensitive payloads (e.g., mRNA) from degradation in biological fluids.
- Precision Targeting: Repeating surface structure enables high-density display of Targeted Modules for precision targeting.
- Payload Versatility: Protected, hollow interior allows for high-efficiency encapsulation of diverse therapeutic cargo (nucleic acids, small molecules, proteins).
VLPs: A Modular Platform for Precision Targeting
Creative Biolabs utilizes the VLP as the core of a Modular Delivery System.
This involves two core, integrated components:
(1) the robust VLP scaffold for stable encapsulation.
(2) the Targeted Module (e.g., aptamers, peptides, scFvs) strategically displayed on the surface.
This modularity allows for the systematic optimization of stability, pharmacokinetics (PK), and precise cell- or tissue-specific targeting, effectively turning a major delivery liability into a controlled therapeutic advantage for payloads like CRISPR-Cas9 components and mRNA.
Comprehensive VLP Engineering and Customization
Our service platform provides end-to-end support for developing VLPs from concept design to scaled production, ensuring optimal performance for your specific research goals.
VLP Expression System
Creative Biolabs maintains diverse host expression platforms to optimize the production yield, purity, and functionality of your VLP based on its structural requirements and post-translational modification needs.
E. coli System
Offers highest yield and lowest cost for robust, non-glycosylated VLP scaffolds.
Yeast System
Cost-effective with post-translational modifications (PTMs) capability, suitable for specific complex VLP.
Eukaryotic Cell Systems (Mammalian/Insect)
Ensures native PTMs and high physiological relevance for complex viral proteins with scalability.
Cell-free System
Rapid, high-throughput VLP production, ideal for screening and fast optimization cycles.
Precision Surface Functionalization
Achieving precise delivery relies on our expertise in VLP surface engineering.
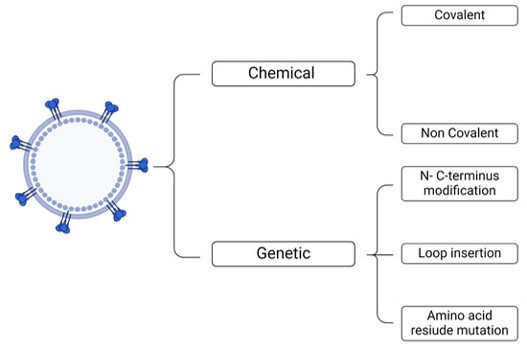 Fig. 2 Methods for functionalizing the surface of VLPs.2,3
Fig. 2 Methods for functionalizing the surface of VLPs.2,3
Chemical Conjugation
Covalent linkage of targeting ligands (e.g., folic acid, probes) to accessible amino acid residues like Cysteine and Lysine on the VLP surface.
Genetic Fusion
Direct incorporation of peptides or small proteins into the VLP capsid structure via recombinant DNA, placing the targeting moiety externally.
Genetic Modification
Fusion of the desired protein/peptide to a full-length viral protein or transmembrane domain to ensure high-density display within the VLP envelope.
Post-Translational Modification
We use optimized host systems to control crucial PTMs (e.g., Glycosylation, Phosphorylation) for optimal VLP function and stability.
Advanced Payload Encapsulation Services
VLPs are uniquely suited to protect and deliver challenging therapeutic payloads that are often unstable in vivo.
Small Molecule Drugs
Cytotoxic Agents, Chemotherapeutics, Hydrophobic Compounds. Optimized loading via clicks chemistry and surface adsorption.
Proteins & Peptides
Enzymes, toxins, and immunogenic epitopes. Display on the VLP surface or internal encapsulation.

Nucleic Acids
mRNA, siRNA, shRNA, DNA Plasmids, CRISPR-Cas9 Components, ASOs. Efficient, high-yield loading via electrostatic interaction, click chemistry, or chemical crosslinking.
Advanced Therapeutics
Protein degraders, immunomodulators. Formulation and stabilization within the VLP core.
Workflow

Applications of VLP in Modern Research
Our custom VLP solutions are designed to unlock potential across the most demanding fields of modern biomedical research:
- Drug Delivery: VLPs are engineered to encapsulate and deliver challenging payloads like mRNA, CRISPR-Cas9 components, and Protein Degraders to specific cells or tissues (e.g., tumors, CNS), maximizing the therapeutic index and improving outcomes.
- Vaccine Development: Leveraging the VLP's innate multivalent architecture, we display antigens in a native-like conformation, significantly enhancing immunogenicity for prophylactic and therapeutic vaccine research against viral pathogens or cancer.
- Membrane Protein Expression: VLPs serve as highly stable, native-conformation platforms for the display of complex membrane proteins (GPCRs, Ion Channels). This is crucial for structural studies and functional screening.
- Antibody Development: We utilize VLPs presenting complex antigens to generate highly specific antibodies (monoclonal or polyclonal) with enhanced binding properties against difficult targets, including those found on the cell surface.
- Bioimaging: VLPs can be readily functionalized with fluorescent or radio-isotopic probes, providing a stable, targeted carrier for in vivo tracking, diagnostic imaging, and research into biodistribution.
Why Choose Creative Biolabs for VLP Development?
True Targeted Delivery Expertise
Our focus is the strategic integration of VLP platforms with advanced Targeted Modules to maximize the therapeutic index, not just commodity VLP production.
Payload Versatility & Protection
Proven track record in successfully encapsulating and protecting the most challenging payloads, including small molecule drugs, proteins/peptides, large mRNA molecules, and complex protein degraders.
Custom Modularity for Precision
Unmatched flexibility in mixing and matching VLP scaffolds, targeting ligands, and host systems to meet unique project specifications, ensuring optimal PK/PD profiles.
Comprehensive Analytical Characterization
Every VLP preparation undergoes rigorous characterization, including TEM, DLS, and functional in vitro assays for targeting efficacy and controlled payload release.
Scalability from Bench to Preclinic
Established, rigorous protocols across multiple host systems (Mammalian, Insect, E. coli) for seamless, compliant transition from research to large-scale pre-clinical production.
Creative Biolabs provides comprehensive, modular VLP development services engineered to solve the most challenging problems in therapeutic delivery. From custom expression and advanced surface functionalization to protected encapsulation of complex payloads (like mRNA and proteins), we offer a full-cycle partnership. Contact us today to discuss your customized VLP development needs and accelerate your next breakthrough.
Related Services
FAQs
What is the primary difference between a VLP and a functional virus?
The key difference is the viral genome. VLPs are composed solely of structural proteins and lack the genetic material necessary for replication, making them entirely non-infectious and safe for use as a drug delivery or vaccine platform.
What is the typical maximum payload size a VLP can carry?
The maximum payload size depends heavily on the VLP scaffold used. Smaller phages (like Qβ) can encapsulate payloads up to ∼1 MDa (sufficient for large mRNA or multiple small molecules), while larger scaffolds offer significantly more capacity. We optimize the VLP choice based on your specific cargo.
Can you customize the surface of the VLP for specific cell targeting?
Yes. Surface functionalization is a core component of our service. We use genetic fusion or chemical conjugation to display Targeted Modules such as aptamers, peptides, or scFvs, directing the VLP exclusively to cells expressing specific surface receptors.
Which expression system is best suited for my VLP project?
The ideal system (Mammalian, Insect, or E. coli) depends on the VLP's complexity. If your VLP requires native eukaryotic PTMs, mammalian or Insect cells are preferred. For non-glycosylated, robust scaffolds requiring high yield and low cost, the E.coli system is typically utilized.
What is the typical turnaround time for a custom VLP production project?
A full VLP development and characterization project typically takes 10 to 15 weeks, depending on the complexity of the scaffold and the required scale. The detailed breakdown is provided in the Workflow section above.
References
- Nooraei, Saghi, et al. "Virus-like particles: preparation, immunogenicity and their roles as nanovaccines and drug nanocarriers." Journal of nanobiotechnology 19.1 (2021): 59. https://doi.org/10.1186/s12951-021-00806-7
- Travassos, Rafael, et al. "Tailored viral-like particles as drivers of medical breakthroughs." International Journal of Molecular Sciences 25.12 (2024): 6699. https://doi.org/10.3390/ijms25126699
- Distributed under an Open Access license CC BY 4.0, without modification.