We offer a bespoke, end-to-end service for developing tailored bioconjugates. This includes custom synthesis of linkers, peptides, or polymers, as well as the optimization of conjugation strategies to achieve your distinct therapeutic objectives.
Bioconjugate Development Services
Are you facing challenges with long drug development cycles, difficulty in protein expression and purification, or complex clinical trials? Creative Biolabs' Bioconjugation Service for target delivery helps you accelerate drug discovery, obtain high-quality recombinant proteins, and streamline clinical trial processes through innovative protein engineering techniques and high-throughput screening platforms.
Overview
Bioconjugation constitutes a deliberate chemical methodology creating covalent bonds between distinct entities to generate novel functional hybrids. This technique has become a cornerstone of modern medicine, enabling the creation of advanced therapeutics with improved properties, such as enhanced specificity, stability, and controlled drug release. The core principle is to leverage the targeting capabilities of a biomolecule (e.g., an antibody or peptide) to deliver a therapeutic or diagnostic payload to a precise location within the body. This approach fundamentally shifts the paradigm from broad-spectrum treatments to highly targeted, precision medicine.
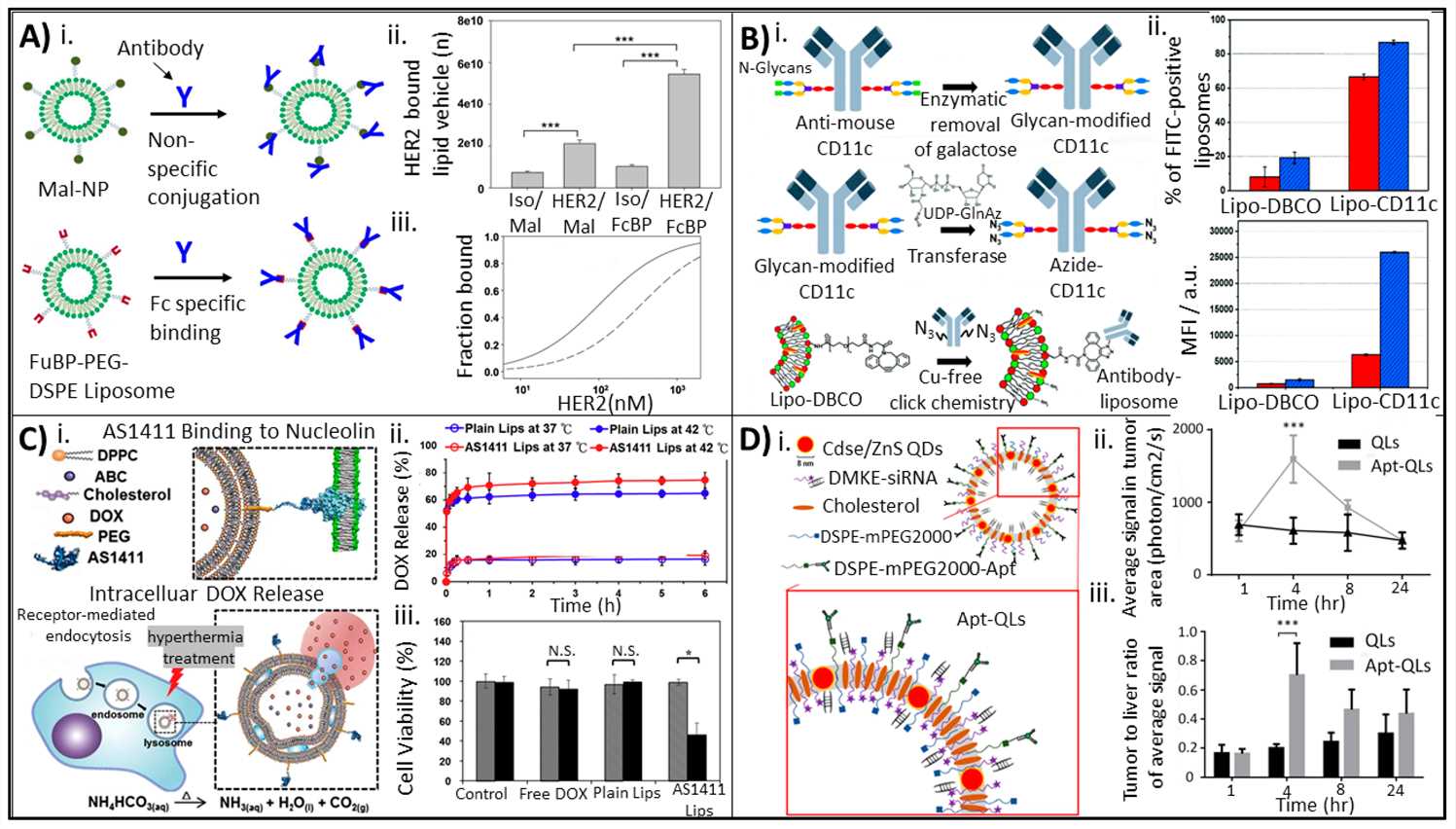 Fig.1 Antibody- and aptamer-based bioconjugation approaches for enhanced therapeutic precision and delivery.1,3
Fig.1 Antibody- and aptamer-based bioconjugation approaches for enhanced therapeutic precision and delivery.1,3
The field has evolved significantly, moving from earlier, less controlled methods to sophisticated techniques that ensure a homogeneous product. This evolution is crucial for developing therapeutic agents that can overcome key biological barriers, such as nuclease degradation, premature clearance, and cellular membrane impermeability, which are often major hurdles for traditional drugs. Bioconjugates are now successfully used in a wide range of applications, including the treatment of various cancers, infectious diseases, and neurodegenerative conditions, as well as in the development of highly effective vaccines and advanced diagnostic tools.
Bioconjugation Services for Targeted Delivery
We specialize in bioconjugation, a key technology for creating highly specific targeted drug delivery systems. Our services enable the precise covalent attachment of therapeutic agents to targeting modules, ensuring that your drugs reach their intended cellular destinations with maximum efficiency and minimal off-target effects.
Our comprehensive mastery encompasses diverse pivotal implementation scenarios. Explore how our Bioconjugation Services can enhance your targeted drug delivery projects. Click below to discover how our solutions can accelerate your research and development.
Bioconjugation Methods
The choice of bioconjugation method is a critical determinant of a conjugate's therapeutic efficacy and stability. The field has advanced beyond "random" conjugation to more controlled and precise approaches.
- Traditional Methods: Early bioconjugation often involved the reaction of a payload with common amino acid residues on a protein, such as lysine or cysteine. While widely used, this method can lead to a heterogeneous mixture of products with varying drug-to-antibody ratios (DAR), which can impact consistency and efficacy.
- Bioorthogonal Chemistry: The development of bioorthogonal chemistry has revolutionized the field. These highly selective reactions, such as the widely used click chemistry, allow for the site-specific conjugation of molecules under mild, physiological conditions. This approach ensures a well-defined and homogeneous product, which is essential for consistent drug performance and simplified regulatory processes.
-
Linker Chemistry: This covalent connector, covalently coupling targeting agents to therapeutic cargo, constitutes a pivotal bioconjugate element.
- Cleavable: These linkers are designed to be stable in systemic circulation but release the payload at the target site in response to specific environmental cues (e.g., changes in pH, or the presence of specific enzymes).
- Non-Cleavable: These linkers permanently attach the drug to the targeting molecule. Active agents are liberated following cellular internalization and lysosomal breakdown within designated cells. The choice between cleavable and non-cleavable linkers is a key strategic decision, balancing the need for stability with the requirement for controlled payload release.
Contact Us About Bioconjugation Services
What We Can Offer?
Creative Biolabs serves as a pioneering force in precision therapeutic conveyance advancement. Our multidisciplinary team of biologists, chemists, and engineers leverages over two decades of collective experience to develop sophisticated bioconjugate solutions that meet your specific project needs. We provide comprehensive support for your R&D pipeline, from initial design to final product validation.
Custom Conjugation Services
Pre-Clinical Validation
Our services include comprehensive in vitro and in vivo testing to assess targeting efficiency, cellular uptake, biodistribution, and therapeutic efficacy. We provide the data you need to confidently advance your drug candidates.
Ready-to-Use Research Tools
We provide a growing catalog of pre-engineered bioconjugation components and kits, including validated linkers, functionalized lipids, and targeted polymers, to accelerate your research and development efforts.
Expert Scientific Partnership
We believe in a collaborative approach. Our team partners with you from the very beginning, offering deep scientific knowledge and state-of-the-art facilities to assist with experimental design, data analysis, and problem-solving at every stage.
Workflow

Why Choose Us?
At Creative Biolabs, our bioconjugation service is built on a foundation of scientific excellence, precision, and client-focused collaboration. Our unique advantages distinguish us as a leader in the field and your ideal partner for therapeutic innovation.
Advanced Methodologies for Precision
We utilize next-generation platforms for molecular coupling and synthesis, engineering homogeneous bioconjugates with a defined drug-to-antibody ratio. This is critical for predictable pharmacokinetics and consistent clinical outcomes.
Bespoke Solutions and Versatility
We offer personalized engineering for oligonucleotides, peptides, and carrier systems. Each component is refined to align with your distinct therapeutic objectives and cellular targets, ensuring a purpose-built solution.
Uncompromising Quality Assurance
Our dedication to methodological precision and rigorous protocols is non-negotiable. We provide a full suite of analytical services to guarantee dependable, consistent, and superior-grade outcomes that meet the most stringent quality and regulatory standards.
Deep-Rooted Expertise
Our multidisciplinary team has over years of collective industry experience in therapeutic delivery. This profound expertise allows us to anticipate challenges and provide unparalleled guidance throughout your project.
Published Data
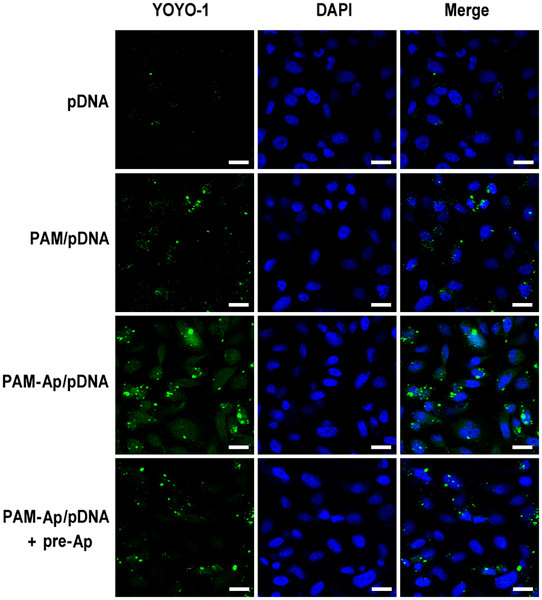 Fig.2 Illustrative confocal microscopy visuals demonstrate cellular internalization of PAM-Ap/pDNA nanoparticles.2,3
Fig.2 Illustrative confocal microscopy visuals demonstrate cellular internalization of PAM-Ap/pDNA nanoparticles.2,3
The research focuses on a novel therapeutic strategy for non-small cell lung cancer (NSCLC) using aptamer-dendrimer bioconjugates for targeted gene delivery. The experiment involved encapsulating MicroRNA-34a (miR-34a), a known tumor suppressor, into S6 aptamer-conjugated dendrimers, creating nanoparticles (PAM-Ap/pMiR-34a NPs). These nanoparticles, with a size of 100-200 nm, were tested on NSCLC cells. Results showed that the aptamer conjugation significantly improved cellular uptake and gene transfection efficiency compared to non-targeted nanoparticles. The nanoparticles effectively delivered the miR-34a plasmid, leading to enhanced regulation of the targeted genes BCL-2 and p53, and effectively inhibiting cell growth, migration, and invasion, while inducing apoptosis in the lung cancer cells. This study demonstrates the potential of aptamer-dendrimer bioconjugates as a highly efficient and specific system for gene delivery, offering a promising new direction for the treatment of challenging cancers like NSCLC.
FAQs
Q: What is the molecular mechanism by which bioconjugation enhances the therapeutic index of a drug compared to a free small-molecule therapeutic?
A: Bioconjugation significantly improves the therapeutic index by leveraging a targeting molecule, such as an antibody, to deliver a potent payload specifically to diseased cells that overexpress a particular receptor. This focused delivery dramatically increases the local concentration of the therapeutic agent at the target site while keeping systemic exposure and off-target toxicity to healthy tissues at a minimum. This precision allows for the use of more potent drugs that would otherwise be too toxic for systemic administration, thereby widening the therapeutic window.
Q: Beyond linker chemistry, what specific analytical and biological assays are used to validate the in vivo stability and functionality of a bioconjugate?
A: A rigorous suite of assays is employed to ensure bioconjugate integrity. We conduct serum stability studies to measure the half-life of the conjugate in a biological matrix. Pharmacokinetic and pharmacodynamic (PK/PD) studies in animal models are used to track the bioconjugate's circulation, distribution, and efficacy. Additionally, in vitro binding assays, such as ELISA or surface plasmon resonance (SPR), are performed to confirm that the targeting molecule's affinity remains uncompromised after conjugation.
Q: What is the process for evaluating the chemical compatibility and functional integrity of a client's proprietary biomolecule or payload within a novel bioconjugation strategy?
A: Our methodology initiates with comprehensive assessment of client-provided compounds. We perform preliminary characterization to understand their chemical properties and structure. This allows us to select an optimal, compatible conjugation chemistry and site-specific strategy that preserves the biomolecule's functional integrity. The process is highly collaborative, with our team designing a tailored workflow and providing continuous analysis to ensure the final bioconjugate retains the desired activity and properties.
Q: How do factors such as biomolecule complexity, linker novelty, and scale of production impact the overall timeline of a bioconjugation project?
A: Project timelines are directly influenced by the technical scope. For example, a novel linker or payload requires additional time for custom synthesis, optimization, and characterization. Similarly, a highly complex biomolecule may necessitate advanced site-specific engineering and purification techniques. Increasing the scale of production from a small-scale research batch to a larger, cGMP-compliant quantity also requires additional time for process development and validation studies, all of which contribute to a longer timeline.
Q: What are the key functional and stability advantages of a covalently linked bioconjugate over a non-covalent drug-targeting complex?
A: The primary advantage of a covalently linked bioconjugate is its enhanced stability and predictable behavior in vivo. The covalent bond prevents premature dissociation of the drug from the targeting molecule in the bloodstream, ensuring the therapeutic payload is delivered specifically to the target cells. In contrast, non-covalent complexes are susceptible to dissociation, which can lead to off-target drug release, reduced efficacy, and increased systemic toxicity. A covalent bond ensures a consistent drug-to-target ratio and a more reliable therapeutic outcome.
At Creative Biolabs, our commitment to scientific excellence and client partnership drives us to deliver the highest quality bioconjugation solutions. We are your trusted collaborators in engineering the next generation of precision therapeutics. Connect with our experts for project-specific consultation and detailed insights.
References
- Almeida, Bethany et al. “Recent Progress in Bioconjugation Strategies for Liposome-Mediated Drug Delivery.” Molecules (Basel, Switzerland) vol. 25,23 5672. 1 Dec. 2020, DOI:10.3390/molecules25235672.
- Wang, Hongmei et al. “Aptamer-Dendrimer Bioconjugates for Targeted Delivery of miR-34a Expressing Plasmid and Antitumor Effects in Non-Small Cell Lung Cancer Cells.” PloS one vol. 10,9 e0139136. 25 Sep. 2015, DOI:10.1371/journal.pone.0139136.
- Distributed under Open Access license CC BY 4.0, without modification.