A comprehensive catalog of pre-validated T cell targeting modules (e.g., specific antibodies, peptides, and aptamers) that are ready for immediate integration into your research and development needs.
T Cell Targeting Module Development Service
Are you currently facing challenges in achieving precise and effective cell-specific delivery for your advanced therapies, particularly those involving T cells? Our T cell Targeting Module Development services at Creative Biolabs help you overcome these hurdles and accelerate your drug discovery and development processes. We enable highly specific payload delivery to T cells through advanced recombinant DNA technology and innovative protein engineering techniques, ensuring enhanced therapeutic efficacy and reduced off-target effects.
Overview
T cells are central players in adaptive immunity, responsible for recognizing and eliminating specific threats such as infected cells and cancer cells. Their unparalleled specificity and cytotoxic potential make them attractive targets for a wide range of therapeutic interventions, including cancer immunotherapy, autoimmune disease treatment, and infectious disease management. T cell targeting module development focuses on creating highly specific components that can direct therapeutic payloads directly to T cells or specific T cell subsets. This precision is crucial to maximize therapeutic efficacy while minimizing off-target effects on healthy cells, a common challenge in systemic therapies.
 Fig.1 3D illustration of a lymphocyte T cell.Distributed under CC BY-SA 4.0, from Wiki, without modification.
Fig.1 3D illustration of a lymphocyte T cell.Distributed under CC BY-SA 4.0, from Wiki, without modification.
These modules often interact with specific surface receptors or markers on T cells. For example, the CD3 complex, a component of the T cell receptor (TCR) complex, is a common target for T cell activation and redirection. Other critical markers include CD4 (found on helper T cells), CD8 (on cytotoxic T cells), and various co-stimulatory or inhibitory receptors like PD-1, CTLA-4, and OX40. Understanding the basic information, structure, and function of these target proteins is fundamental for designing effective targeting modules. For instance, engaging CD3 can lead to T cell activation, while targeting immune checkpoints like PD-1 can reinvigorate exhausted T cells in the tumor microenvironment. The related signaling pathways, such as the TCR signaling cascade, NF-κB, and MAPK pathways, are critical for T cell activation, proliferation, and differentiation, all of which can be modulated by targeted delivery. Diseases such as cancer, autoimmune disorders (e.g., rheumatoid arthritis, multiple sclerosis), and chronic viral infections often involve dysregulated T cell responses, highlighting the immense potential for targeted T cell therapies.
Creative Biolabs' T Cell Targeting Solution
Creative Biolabs' precision delivery platform is built around T cell targeting modules. These meticulously engineered components integrate into various delivery systems like lipid nanoparticles (LNPs), liposomes, exosomes, or viral vectors. These systems are functionalized with specific ligands that recognize and bind to receptors or surface markers on T cells or their specific subsets.
T cell uptake primarily involves active receptor-mediated endocytosis once targeted modules engage with surface markers. This binding triggers uptake, ensuring selective delivery of payloads—genes, small molecules, or proteins. Our modular design offers unparalleled flexibility, allowing customization for diverse research and therapeutic goals, from enhancing adoptive cell therapies to precisely delivering immunomodulatory agents.
Passive Targeting
While active targeting is our main focus, some larger delivery systems can passively accumulate in highly permeable tissues (e.g., inflamed or tumor tissues) due to the Enhanced Permeability and Retention (EPR) effect. T cells might then interact with these. However, for highly specific T cell engagement, active targeting remains paramount.
Active Targeting
This cornerstone of our T cell targeting module development involves functionalizing delivery systems with specific ligands that bind to overexpressed receptors on T cells or particular T cell subsets. This ensures precise cellular recognition and uptake, leading to highly efficient and selective delivery of the therapeutic cargo.
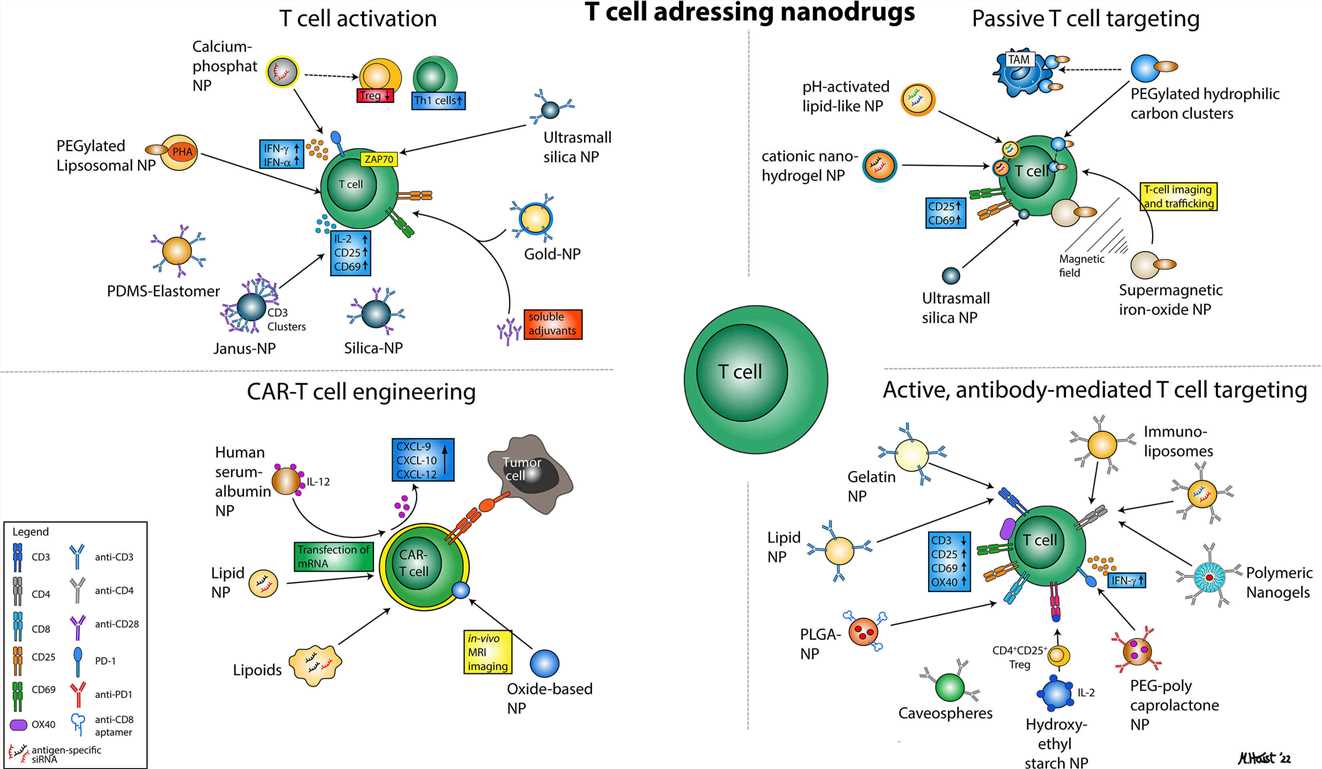 Fig.2 Illustration of various nanoparticular approaches to address T cells for immunotherapy.1,3
Fig.2 Illustration of various nanoparticular approaches to address T cells for immunotherapy.1,3
T cell Targeting Module
Creative Biolabs' diverse library of targeted modules provides unparalleled flexibility in achieving precise T cell delivery. Each module type offers distinct advantages in terms of specificity, stability, and ease of conjugation to our various delivery systems. Our T cell targeting module is a core component of our advanced delivery system, engineered for precise interaction with T cell surface receptors. It typically consists of a high-affinity ligand (e.g., a peptide, antibody fragment, or carbohydrate) specifically chosen for its binding to a target marker like CD3, CD4, or CD8, conjugated to a linker that facilitates stable attachment to the delivery vehicle (liposomes, exosomes, micelles, polymer nanoparticles, etc.). This modular approach allows for flexibility and optimization across various therapeutic applications.
Here are various ligand types used in T cell targeting:
| Ligand Type | Mechanism of Action | Targeted Marker(s) | Advantages/Application |
|---|---|---|---|
| Antibodies | Highly specific proteins that bind to unique antigens on the cell surface. | CD3, CD4, CD8, PD-1, CTLA-4, OX40, GITR, CD28, CD40L | Unparalleled precision and intense binding selectivity. Configurable as intact immunoglobulins, fragments (Fab, scFv), or bispecific formats for simultaneous engagement. |
| Peptide | Short amino acid sequences designed to bind to specific receptors or unique protein conformations. | Various tumor-associated antigens (when presented on MHC), specific T cell activation markers. | Compact structure, effective biodistribution, extensive adaptability, and minimal immune activation. Economical production. |
| Carbohydrates | Glycans that specifically interact with lectin-like receptors on T cells. | CD2, CD7 | May regulate T lymphocyte stimulation, attachment, and trafficking. Potentially reduced immunogenic potential versus proteins. |
| Aptamers | Single-stranded DNA or RNA oligonucleotides that fold into unique 3D structures to bind specific molecular targets. | CD3, CD4, CD8, PD-1 | Superior molecular recognition, intense binding force, diminutive scale, low immune reactivity, and facile chemical production/alteration. Effortlessly coupled to nanocarriers. Provide antibody alternatives possessing enhanced tissue permeation. |
| Other | Molecules designed to interact with intracellular or surface receptors, or growth factors/signaling molecules. | Various intracellular signaling molecules, cytokine receptors (e.g., IL-2R, IL-15R) | Can adjust T cell activity directly or indirectly. Low-MW compounds traverse membranes targeting internal pathways. |
Contact Us About T cell Targeting Module
What We Can Offer?
Creative Biolabs is uniquely positioned at the forefront of targeted drug delivery innovation, with a focus on T cell targeting. Our team of expert biologists, chemists, and engineers brings over two decades of collective experience in developing sophisticated delivery solutions.
We offer:
Ready-to-Use Products
Customized Services
Our bespoke service allows us to develop tailored T cell targeting modules from concept to validation, precisely meeting your project's unique specifications. This includes custom ligand design, synthesis, and optimization for specific T cell subsets or therapeutic applications.
Conjugation Services
Expertise in conjugating selected T cell-specific ligands to various delivery platforms, including nanoparticles, liposomes, exosomes, and viral vectors.
Pre-Clinical Validation
Robust in vitro and in vivo testing to assess targeting efficiency, cellular uptake, T cell activation, biodistribution, and therapeutic efficacy in relevant disease models.
Comprehensive Scientific Support
Partner with us to leverage our deep scientific knowledge, state-of-the-art facilities, and rigorous quality control for your T cell targeting projects, from experimental design to data analysis and interpretation.
Workflow

Why Choose Us?
Partner with Creative Biolabs for accelerated T-cell therapy development, achieving enhanced efficacy and reduced off-target effects. Our innovation ensures your therapeutic agents precisely target T cells, opening new treatment possibilities.
Proven Expertise
Our specialized team offers deep scientific knowledge and a successful track record in targeted delivery and T-cell module development.
Innovative Technology
We utilize state-of-the-art platforms for module synthesis and characterization, ensuring high-quality, reproducible results.
Tailored Customization
We provide highly customized ligand design and delivery system optimization to perfectly match your unique therapeutic goals.
Rigorous Quality
Our commitment to stringent quality control guarantees reliable, high-quality outcomes for your critical projects.
Published Data
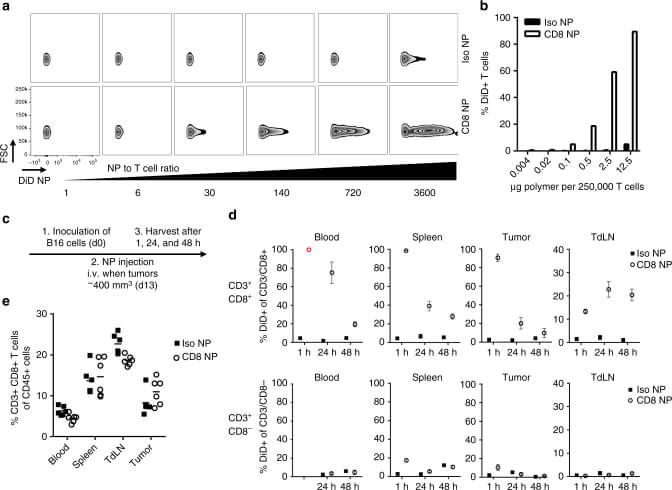 Fig.3 CD8a-targeting nanoparticles bind to T cell in vitro and in vivo.2,3
Fig.3 CD8a-targeting nanoparticles bind to T cell in vitro and in vivo.2,3
In the experiments, the researchers developed nanoparticles capable of binding to specific T cell subsets, such as CD8+ T cells and PD-1-expressing T cells, and loaded them with various small molecule drugs for sustained release. A significant finding was that the targeted delivery of a TGF-beta signaling inhibitor (SD-208) specifically to PD-1-expressing T cells led to a substantial delay in tumor growth and extended survival in tumor-bearing mice. This effect was not observed when the free drug was administered systemically at comparable doses. Furthermore, the platform enabled the PD-1-targeted delivery of a TLR7/8 agonist, which resulted in an increase in tumor-infiltrating CD8+ T cells and sensitized the tumors to subsequent anti-PD-1 therapy. These results highlight that the targeted delivery of immunomodulatory compounds to defined subsets of endogenous leukocytes offers a superior approach compared to systemic administration of free drugs, presenting a novel strategy to improve patient response rates in cancer immunotherapy.
FAQs
Q: How do T cell targeting modules ensure specificity, and what are the benefits?
A: T cell targeting modules are engineered with specific ligands (e.g., antibodies, peptides, aptamers) that bind unique surface markers on T cells or their subsets. This ensures precise payload delivery to intended cells, minimizing off-target effects. Benefits include enhanced therapeutic efficacy, reduced systemic toxicity, and targeted T cell modulation without broad suppression.
Q: What types of payloads can be delivered using these targeting modules?
A: Our versatile modules deliver diverse payloads: nucleic acids (e.g., mRNA, siRNA), small molecules, proteins, enzymes, and imaging agents. Their modular design integrates flexibly with delivery systems (nanoparticles, liposomes, viral vectors) for precise T cell delivery.
Q: Are these targeting modules suitable for both in vitro and in vivo applications?
A: Yes, our T cell targeting modules are validated for both in vitro and in vivo use. In vitro, they enable precise cell studies and therapeutic optimization. In vivo, they facilitate targeted drug/gene delivery and adoptive cell therapy in preclinical models, showing enhanced biodistribution and outcomes.
Q: What are the key advantages of using targeted modules over traditional systemic therapies for T cell modulation?
A: Key advantages are improved specificity and reduced systemic side effects. Systemic therapies often harm healthy cells, causing toxicities. Targeted modules concentrate agents on T cells, minimizing exposure to other tissues. This yields higher therapeutic indexes, more effective treatment, and lower dosages, enhancing safety.
Q: How can these modules be customized for specific T cell subsets or disease contexts?
A: Customization is a core strength: we design ligands binding unique markers on specific T cell subsets (e.g., effector, regulatory, exhausted) or T cells in disease microenvironments (e.g., TILs). This involves advanced protein engineering, high-throughput screening, and rational design.
Creative Biolabs leads T cell Targeting Module Development, empowering biopharmaceutical researchers. Our excellence and state-of-the-art platforms ensure highly specific, efficient, and reliable modules. We provide ready-to-use products, custom development, and preclinical validation to accelerate projects, enhance efficacy, and minimize off-target effects. Partner with us to unlock T cell therapy potential and advance precision medicine.
Contact Our Team for More Information and to Discuss Your Project.
References
- Haist, Maximilian et al. "Nanodrugs Targeting T Cells in Tumor Therapy." Frontiers in immunology vol. 13 912594. 25 May. 2022, DOI:10.3389/fimmu.2022.912594.
- Schmid, Daniela et al. "T cell-targeting nanoparticles focus delivery of immunotherapy to improve antitumor immunity." Nature communications vol. 8,1 1747. 23 Nov. 2017, DOI:10.1038/s41467-017-01830-8.
- Distributed under Open Access license CC BY 4.0, without modification.



