The landscape of gene therapy and mRNA vaccine development is rapidly evolving, yet it presents significant challenges in achieving safe and effective delivery of delicate nucleic acid payloads. Overcoming biological barriers and ensuring payload stability are critical hurdles that demand innovative solutions. Lipid nanoparticles (LNPs) have emerged as a revolutionary delivery system, offering a promising avenue to address these complexities. At Creative Biolabs, we specialize in providing advanced and ready-to-use LNP products, empowering researchers to push the boundaries of targeted drug delivery in this crucial field.
Overview
At the forefront of nucleic acid delivery, LNPs are sophisticated, self-assembling systems engineered from a precise blend of ionizable lipids, helper lipids, cholesterol, and PEGylated lipids. As a leading non-viral vector platform, LNPs are meticulously designed to encapsulate and shield delicate therapeutic payloads—such as mRNA, siRNA, and RNA-guided gene editing systems—from degradation within complex biological environments. By enabling the precise transport and efficient intracellular delivery of these molecules, LNPs effectively overcome critical biological barriers, including enzymatic breakdown in the bloodstream and the challenge of cellular membrane impermeability. This unique capability establishes LNPs as indispensable tools for pioneering research and development in gene therapy, mRNA vaccines, RNAi therapeutics, and advanced cell therapies.
Biocompatibility and Biodegradability
Composed of naturally occurring or synthetic lipids, LNPs exhibit favorable biocompatibility and are designed to degrade safely within the body, minimizing long-term toxicity.
Tunable Surface Chemistry
The surface of LNPs can be readily modified with targeting ligands (e.g., antibodies, peptides, aptamers) to achieve cell-specific or tissue-specific delivery, enhancing therapeutic precision.
Sustained and Controlled Release
The lipid bilayer can be engineered to release its payload over a prolonged period, prolonging the drug's half-life and achieving sustained therapeutic effects.
Versatile Payload Compatibility
LNPs can encapsulate a broad spectrum of therapeutic agents, including various nucleic acids (mRNA, siRNA, DNA plasmids, CircRNA, ASO), small molecule drugs, and certain proteins/peptides.
Explore Our Product Portfolio
View All Products
Creative Biolabs offers a diverse range of ready-to-use LNP products meticulously designed to meet the varied and evolving needs of researchers.
Empty LNP
Explore pre-formulated liposomes with agents like clodronate or doxorubicin for preclinical research.
Fluorescent LNPs
Ideal for in vitro and in vivo tracking and visualization of LNP delivery, these LNPs streamline studies of cellular uptake and biodistribution.
mRNA & CAR mRNA-LNP
Pre-formulated LNPs encapsulating mRNA or CAR mRNA, prepared for immediate use in Gene Therapy and Cell Therapy applications, including CAR-T development.
siRNA-LNP
Optimized LNPs for the efficient delivery of siRNA, enabling robust gene silencing in RNAi therapy research.
CircRNA-LNP
Novel LNP formulations engineered for the stable and efficient delivery of circular RNA, opening new avenues in advanced drug delivery.
Pep-LNP & Ab-LNP
Optimized LNPs for the efficient delivery of siRNA, enabling robust gene silencing in RNAi therapy research.
In addition to our comprehensive range of LNP products, we also provide high-quality LNP-related raw materials. These include essential components such as ionizable lipids, PEGylated lipids, helper lipids, and cholesterol, empowering researchers with the foundational elements for their custom LNP development. Contact us today to discuss your specific raw material needs and empower your custom LNP development.
Need a Custom Solution?
Request a Custom Service Consultation
Should your specific application necessitate a unique product, or if a novel combination of LNP products and functionalities is required, our custom development services provide the definitive answer. We engage in collaborative partnerships to transform your innovative concepts into tangible, high-performance delivery solutions.
Nucleic Acid Synthesis & Modification
Specialized services for the synthesis of high-quality nucleic acids, including various modifications such as Cap1, N1-methyl-pseudouridine, and 5-methoxyuridine, to optimize stability and translation efficiency for LNP encapsulation.
Customized LNP Formulation
Clients can specify their desired LNP formula or request custom formulations developed based on specific literature references, ensuring precise control over composition.
Customizable LNP Concentration
While our default LNP concentration is 0.2 mg/ml, clients can specify their desired concentration to meet the precise requirements of their experimental setup.
High-Throughput Screening of LNP
Utilizing advanced screening platforms to rapidly identify optimal LNP formulations for specific payloads and cell types, accelerating LNP development.
Custom Payload Encapsulation
Specialized services for encapsulating a wide array of therapeutic payloads, including mRNA and other nucleic acid drugs.
Conjugation Strategy Design
Expert guidance and execution for the attachment of targeting ligands (e.g., aptamers, peptides, antibodies) for enhanced Targeted delivery, encompassing Aptamer-conjugated LNP and Peptide-conjugated LNP.
Characterization
Rigorous analytical services (e.g., size, PDI, zeta potential, drug loading) for LNP characterization to comprehensively evaluate your LNP formulations.
Targeting Verification
Comprehensive support for assessing the efficacy and safety of your custom delivery systems, including in vitro and in vivo studies across various cell and animal models, assisting clients in biological distribution and other research objectives, and ensuring high transfection efficiency in cell transfection.
Case Study
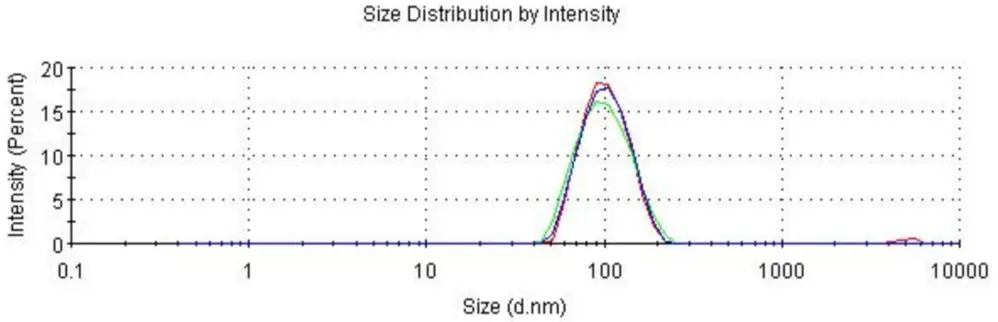
Fluc mRNA-LNP
-
Size: 99.07 nm
-
PDI: 0.149
-
Zeta Potential: -5.36 mV
-
Encapsulation Efficiency: 99.95%
Applications of LNP
-
mRNA Vaccines: LNPs form the foundational backbone for the development of highly effective mRNA vaccine candidates for infectious diseases (e.g., COVID-19) and are actively being explored for cancer immunotherapies.
-
Gene Therapy: Facilitating the intracellular delivery of genetic material for precise genomic modifications in therapeutic applications.
-
RNAi Therapy: Enabling the targeted delivery of siRNA and shRNA molecules for gene silencing in diverse disease contexts.
-
Cell Therapy: Crucial for delivering genetic payloads for cell transfection in applications such as CAR-T cell engineering, thereby ensuring high transfection efficiency.
-
Oncology Drug Delivery: Facilitating the precise delivery of chemotherapeutics, immunomodulators, or nucleic acids specifically to tumor cells, which enhances efficacy and mitigates systemic toxicity, including Oncology drug delivery LNP.
-
Nucleic Acid Delivery: Providing a robust platform for the efficient delivery of various nucleic acids, such as mRNA, siRNA, circRNA, and ASO, for diverse therapeutic strategies.
-
Therapeutic LNP: Utilizing LNPs for a broad spectrum of therapeutic applications beyond nucleic acids, encompassing protein and peptide delivery.
Why Choose Creative Biolabs?
-
Deep Scientific Expertise: With over 20 years of experience, our team possesses a profound understanding of liposome chemistry, targeted delivery, and therapeutic applications to guide your project to success.
-
Comprehensive Portfolio: From high-purity raw materials like Ionizable Lipids and PEGylated lipids to diverse Pre-formulated LNP products (e.g., mRNA-LNP, siRNA-LNP, Pep-LNP), we offer a complete suite of solutions to meet your specific research needs.
-
LNP Amplification Production: Scalable production services for LNPs, ranging from research-grade quantities to larger batches required for preclinical studies.
-
Quality Assurance & Reliability: Every product and service is meticulously validated to ensure consistent performance and reliability, giving you confidence in your results.
-
Innovation-Driven Approach: We continuously invest in R&D to bring you the latest advancements in LNP technology.
-
24-Hour Technical Support: Benefit from our 24-hour technical support to address your inquiries promptly and ensure seamless progress in your research, minimizing downtime.
Product Ordering Process
FAQs
What types of payloads can Creative Biolabs' LNP products deliver?
Our LNP products accommodate a vast array of payloads, including mRNA, siRNA, CircRNA, ASOs, DNA plasmids, CRISPR components, small molecules, proteins, and peptides, supporting diverse Drug encapsulation LNP needs.
How does Creative Biolabs ensure product quality?
Creative Biolabs adheres to stringent quality control throughout manufacturing. Our LNP products undergo rigorous LNP characterization (size, PDI, zeta potential, encapsulation efficiency) to guarantee high purity, stability, and consistent performance.
What is the typical turnaround time for custom LNP orders?
Turnaround times vary based on complexity and scale. Please contact our technical support team with your specific requirements for a detailed timeline estimate.
Do you offer technical support for LNP product usage?
Yes, our experienced scientific team provides comprehensive technical support to assist with product selection, experimental design, and troubleshooting to ensure successful LNP applications.
Can Creative Biolabs assist with in vivo studies?
We offer in vitro and in vivo targeting verification services, including studies across various cell and animal models, to help clients assess the efficacy and biodistribution of their custom LNP delivery systems.
For Research Use Only.
Payload Type: mRNA
Module Type: Unconjugation
Modification: Unmodified; N1-methyl-pseudouridine; 5-methoxyuridine
Application: Control; Targeted Delivery; Tracking and Imaging; Gene Therapy
Payload Type: mRNA
Module Type: Peptide
Modification: Unmodified; N1-methyl-pseudouridine; 5-methoxyuridine
Application: Control; Targeted Delivery; Gene Therapy
Payload Type: mRNA
Module Type: Peptide
Modification: Unmodified; N1-methyl-pseudouridine; 5-methoxyuridine
Application: Control; Targeted Delivery; Gene Therapy
Payload Type: mRNA
Module Type: Unconjugation
Modification: Unmodified; N1-methyl-pseudouridine; 5-methoxyuridine
Application: Control; Targeted Delivery; Tracking and Imaging; Gene Therapy
Payload Type: mRNA
Module Type: Unconjugation
Modification: Unmodified; N1-methyl-pseudouridine; 5-methoxyuridine
Application: Control; Targeted Delivery; Tracking and Imaging; Gene Therapy
Payload Type: CAR mRNA
Module Type: Unconjugation
Modification: Unmodified
Application: Control; Gene Therapy; CAR-T
Payload Type: CAR mRNA
Module Type: Unconjugation
Modification: Unmodified
Application: Control; Gene Therapy; CAR-T
Payload Type: mRNA
Module Type: Peptide
Modification: Unmodified; N1-methyl-pseudouridine; 5-methoxyuridine
Application: Control; Targeted Delivery; Gene Therapy
Payload Type: CAR mRNA
Module Type: Unconjugation
Modification: Unmodified
Application: Control; Gene Therapy; CAR-T
Payload Type: CAR mRNA
Module Type: Unconjugation
Modification: Unmodified
Application: Control; Gene Therapy; CAR-T
Payload Type: mRNA
Module Type: Peptide
Modification: Unmodified; N1-methyl-pseudouridine; 5-methoxyuridine
Application: Control; Targeted Delivery; Gene Therapy
Payload Type: CircRNA
Module Type: Unconjugation
Modification: Unmodified
Application: Control; Targeted Delivery; Tracking and Imaging; Gene Therapy
Payload Type: mRNA
Module Type: Peptide
Modification: Unmodified; N1-methyl-pseudouridine; 5-methoxyuridine
Application: Control; Targeted Delivery; Gene Therapy
Payload Type: mRNA
Module Type: Peptide
Modification: Unmodified; N1-methyl-pseudouridine; 5-methoxyuridine
Application: Control; Targeted Delivery; Gene Therapy
Payload Type: Empty
Module Type: Unconjugation
Application: Control; Targeted Delivery; Tracking and Imaging; Gene Therapy
Payload Type: mRNA
Module Type: Unconjugation
Modification: Unmodified; N1-methyl-pseudouridine; 5-methoxyuridine
Application: Control; Targeted Delivery; Tracking and Imaging; Gene Therapy
Payload Type: CircRNA
Module Type: Unconjugation
Modification: Unmodified
Application: Control; Targeted Delivery; Tracking and Imaging; Gene Therapy
Payload Type: mRNA
Module Type: Unconjugation
Modification: Unmodified; N1-methyl-pseudouridine; 5-methoxyuridine
Application: Control; Targeted Delivery; Tracking and Imaging; Gene Therapy
Payload Type: mRNA
Module Type: Peptide
Modification: Unmodified; N1-methyl-pseudouridine; 5-methoxyuridine
Application: Control; Targeted Delivery; Gene Therapy
Payload Type: CAR mRNA
Module Type: Unconjugation
Modification: Unmodified
Application: Control; Gene Therapy; CAR-T
Payload Type: mRNA
Module Type: Peptide
Modification: Unmodified; N1-methyl-pseudouridine; 5-methoxyuridine
Application: Control; Targeted Delivery; Gene Therapy
Payload Type: mRNA
Module Type: Peptide
Modification: Unmodified; N1-methyl-pseudouridine; 5-methoxyuridine
Application: Control; Targeted Delivery; Gene Therapy
Payload Type: CAR mRNA
Module Type: Unconjugation
Modification: Unmodified
Application: Control; Gene Therapy; CAR-T
Payload Type: mRNA
Module Type: Peptide
Modification: Unmodified; N1-methyl-pseudouridine; 5-methoxyuridine
Application: Control; Targeted Delivery; Gene Therapy
Payload Type: CAR mRNA
Module Type: Unconjugation
Modification: Unmodified
Application: Control; Gene Therapy; CAR-T
Payload Type: mRNA
Module Type: Peptide
Modification: Unmodified; N1-methyl-pseudouridine; 5-methoxyuridine
Application: Control; Targeted Delivery; Gene Therapy
Payload Type: CAR mRNA
Module Type: Unconjugation
Modification: Unmodified
Application: Control; Gene Therapy; CAR-T
Payload Type: mRNA
Module Type: Peptide
Modification: Unmodified; N1-methyl-pseudouridine; 5-methoxyuridine
Application: Control; Targeted Delivery; Gene Therapy
Payload Type: mRNA
Module Type: Antibody
Modification: Unmodified; N1-methyl-pseudouridine; 5-methoxyuridine
Application: Control; Targeted Delivery; Gene Therapy
Payload Type: mRNA
Module Type: Peptide
Modification: Unmodified; N1-methyl-pseudouridine; 5-methoxyuridine
Application: Control; Targeted Delivery; Gene Therapy
Payload Type: CAR mRNA
Module Type: Unconjugation
Modification: Unmodified
Application: Control; Gene Therapy; CAR-T
Payload Type: CAR mRNA
Module Type: Unconjugation
Modification: Unmodified
Application: Control; Gene Therapy; CAR-T
Payload Type: CAR mRNA
Module Type: Unconjugation
Modification: Unmodified
Application: Control; Gene Therapy; CAR-T
Payload Type: mRNA
Module Type: Peptide
Modification: Unmodified; N1-methyl-pseudouridine; 5-methoxyuridine
Application: Control; Targeted Delivery; Gene Therapy
Payload Type: CAR mRNA
Module Type: Unconjugation
Modification: Unmodified
Application: Control; Gene Therapy; CAR-T
Payload Type: CAR mRNA
Module Type: Unconjugation
Modification: Unmodified
Application: Control; Gene Therapy; CAR-T
Payload Type: mRNA
Module Type: Peptide
Modification: Unmodified; N1-methyl-pseudouridine; 5-methoxyuridine
Application: Control; Targeted Delivery; Gene Therapy
Payload Type: mRNA
Module Type: Peptide
Modification: Unmodified; N1-methyl-pseudouridine; 5-methoxyuridine
Application: Control; Targeted Delivery; Gene Therapy
Payload Type: CAR mRNA
Module Type: Unconjugation
Modification: Unmodified
Application: Control; Gene Therapy; CAR-T
Payload Type: mRNA
Module Type: Peptide
Modification: Unmodified; N1-methyl-pseudouridine; 5-methoxyuridine
Application: Control; Targeted Delivery; Gene Therapy
Payload Type: mRNA
Module Type: Peptide
Modification: Unmodified; N1-methyl-pseudouridine; 5-methoxyuridine
Application: Control; Targeted Delivery; Gene Therapy
Payload Type: mRNA
Module Type: Peptide
Modification: Unmodified; N1-methyl-pseudouridine; 5-methoxyuridine
Application: Control; Targeted Delivery; Gene Therapy
Payload Type: mRNA
Module Type: Peptide
Modification: Unmodified; N1-methyl-pseudouridine; 5-methoxyuridine
Application: Control; Targeted Delivery; Gene Therapy
Payload Type: CAR mRNA
Module Type: Unconjugation
Modification: Unmodified
Application: Control; Gene Therapy; CAR-T
Payload Type: mRNA
Module Type: Peptide
Modification: Unmodified; N1-methyl-pseudouridine; 5-methoxyuridine
Application: Control; Targeted Delivery; Gene Therapy
Payload Type: CAR mRNA
Module Type: Unconjugation
Modification: Unmodified
Application: Control; Gene Therapy; CAR-T
Payload Type: mRNA
Module Type: Antibody
Modification: Unmodified; N1-methyl-pseudouridine; 5-methoxyuridine
Application: Control; Targeted Delivery; Gene Therapy
Payload Type: mRNA
Module Type: Unconjugation
Modification: Unmodified; N1-methyl-pseudouridine; 5-methoxyuridine
Application: Control; Targeted Delivery; Tracking and Imaging; Gene Therapy
Payload Type: mRNA
Module Type: Unconjugation
Modification: Unmodified; N1-methyl-pseudouridine; 5-methoxyuridine
Application: Control; Targeted Delivery; Tracking and Imaging; Gene Therapy
Payload Type: CAR mRNA
Module Type: Unconjugation
Modification: Unmodified
Application: Control; Gene Therapy; CAR-T









 Datasheet
Datasheet.png)