A comprehensive catalog of pre-formulated Module Delivery Systems (liposomes, exosomes, LNPs, polymeric nanoparticles) and validated Targeted Modules (aptamers, peptides, etc.) for B cell engagement, ready for your R&D.
B Cell Targeting Module Development Service
Are you currently facing challenges in achieving precise cellular targeting for your autoimmune disease therapies, oncology treatments, or vaccine development? Creative Biolabs' B Cell Targeting Module Development Services helps you overcome off-target effects and enhance therapeutic efficacy by delivering payloads directly to specific B cell populations through advanced ligand engineering and sophisticated delivery platforms.
Overview
B lymphocytes, or B cells, are pivotal components of the adaptive immune system, primarily recognized for their role in producing antibodies. However, their functions extend far beyond antibody secretion, encompassing antigen presentation, cytokine production, and the regulation of immune responses. Given their multifaceted involvement in various physiological and pathological processes, including autoimmune diseases (e.g., rheumatoid arthritis, systemic lupus erythematosus), lymphomas, leukemias, and even infectious diseases, B cells represent highly attractive and therapeutically relevant targets.
Targeting specific B cell populations or their functions offers immense potential for precise disease intervention. For instance, depleting pathogenic B cell subsets can mitigate autoimmune responses, while delivering specific payloads to cancerous B cells can enhance anti-tumor efficacy. The development of B cell targeting modules involves engineering molecular constructs that can selectively recognize and bind to unique surface markers expressed on B cells, facilitating the precise delivery of therapeutic agents (e.g., small molecule drugs, nucleic acids, toxins) or diagnostic probes. This approach aims to maximize therapeutic benefit while minimizing off-target effects on healthy cells, thereby improving drug safety and efficacy profiles.
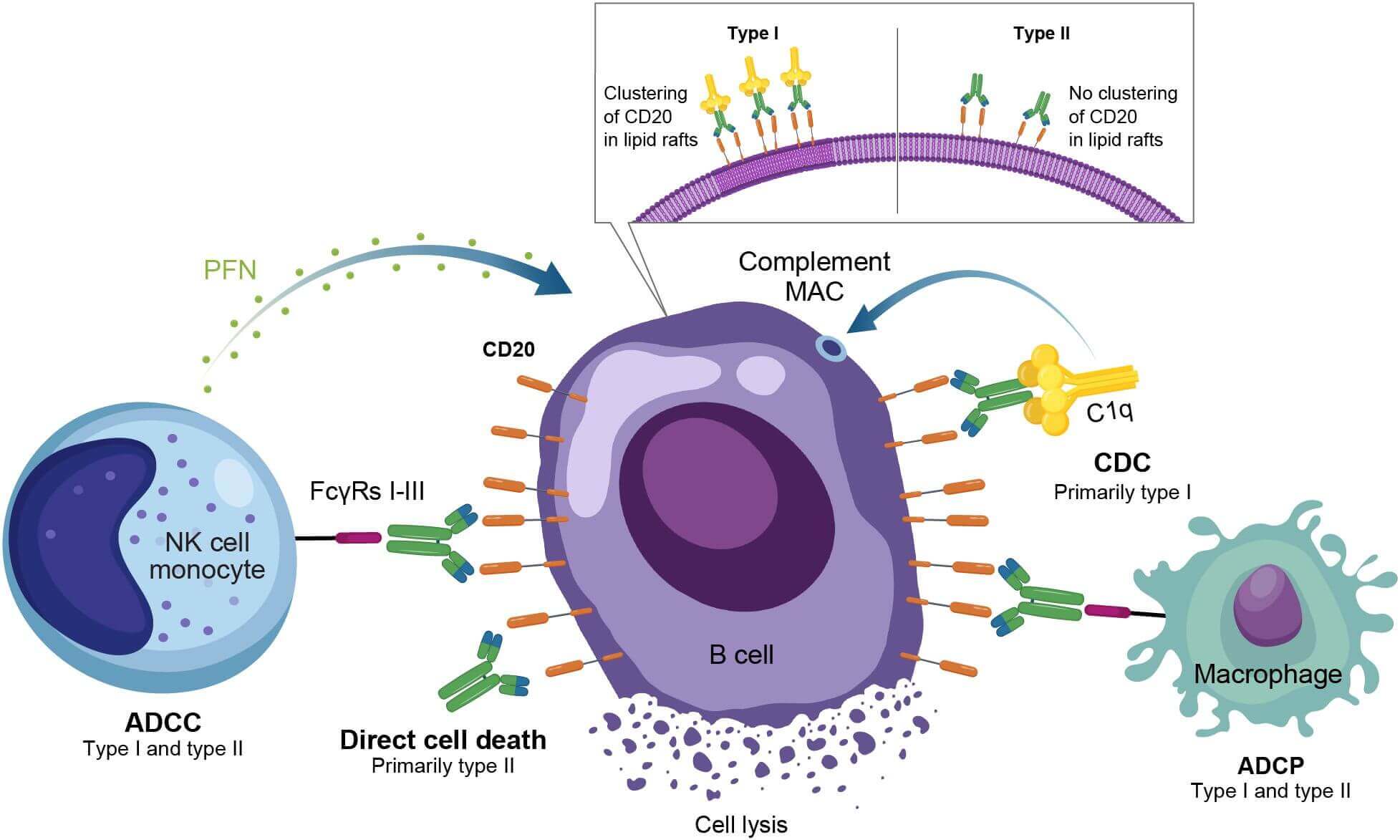 Fig.1 Overview of the mechanism of action for anti-CD20 monoclonal antibodies in autoimmune disease.1,3
Fig.1 Overview of the mechanism of action for anti-CD20 monoclonal antibodies in autoimmune disease.1,3
Creative Biolabs' B Cell Targeting Solution
At Creative Biolabs, our B cell targeting modules form the core of our accurate delivery approach. These expertly designed elements seamlessly integrate into diverse carriers, such as LNPs, liposomes, and exosomes, by incorporating specialized ligands that recognize B cell surface indicators. B cell internalization generally occurs through passive nanoparticle accumulation, followed by active receptor-driven endocytosis once ligands engage, guaranteeing targeted cargo delivery. This adaptable framework allows for versatile tailoring to various research and therapeutic objectives.
Passive Targeting
Nanoscale particles (100-200 nm) gather without active guidance in impaired blood vessels, like those found in tumors or inflamed lymphatic regions, through the Enhanced Permeability and Retention (EPR) phenomenon. B lymphocytes within these locales can subsequently absorb these accumulated particles, though this method offers less selectivity than active targeting.
Active Targeting
A pivotal aspect of our B cell targeting module creation, active targeting entails modifying delivery vehicles with specialized ligands that attach to receptors highly expressed on B lymphocyte membranes or their subpopulations. This guarantees accurate cellular identification and exceptionally selective therapeutic cargo delivery.
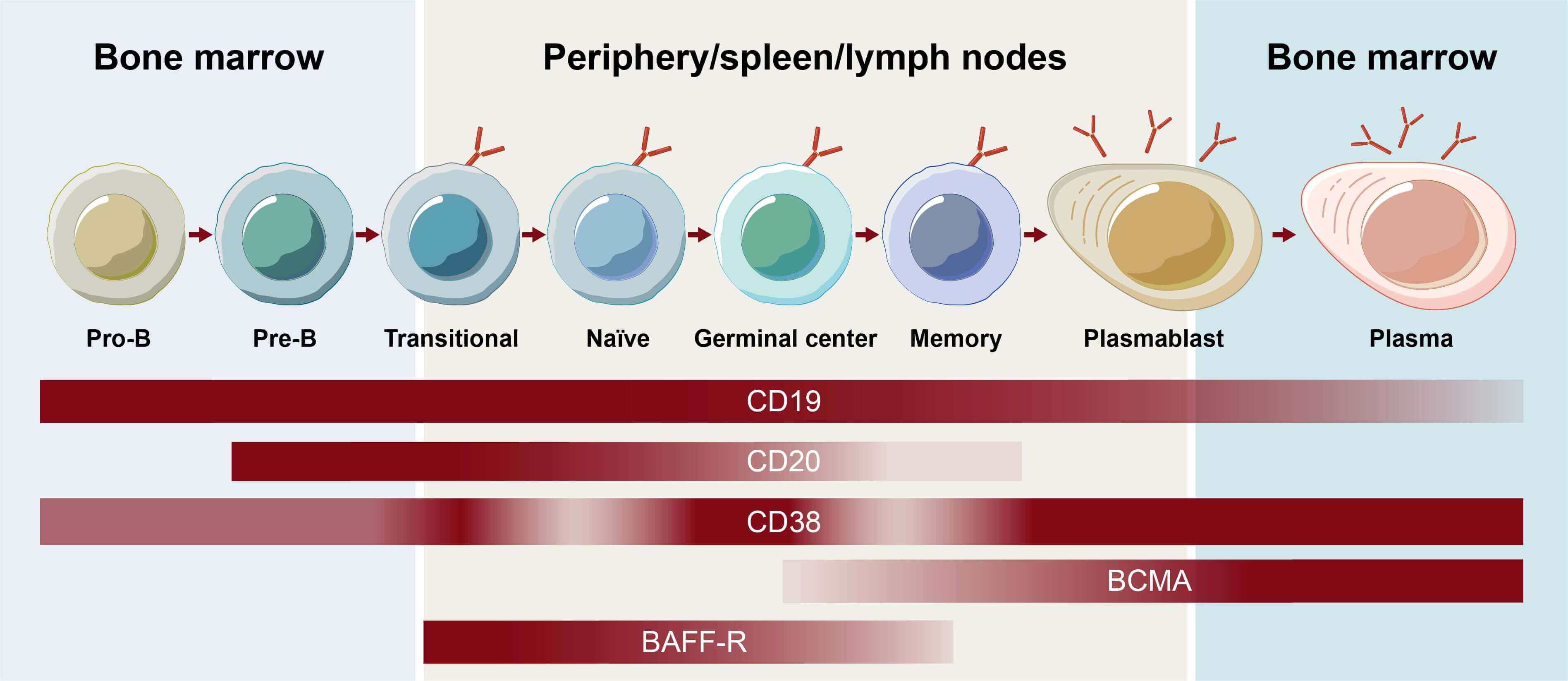 Fig.2 Receptor presence across B-lymphocyte subsets during developmental maturation.1,3
Fig.2 Receptor presence across B-lymphocyte subsets during developmental maturation.1,3
B Cell Targeting Module
The cornerstone of Creative Biolabs' precision drug delivery lies in our advanced B cell targeting modules. These modules are designed to steer our delivery systems straight to particular types of B cells, sometimes even specific subsets (e.g., naive, memory, plasma cells). This selective recognition is achieved through high-affinity binding to unique surface markers expressed on the target cells, ensuring that therapeutic payloads accumulate precisely where they are needed, minimizing off-target effects and maximizing therapeutic efficacy. Creative Biolabs' diverse library of targeted modules provides unparalleled flexibility in achieving precise B cell delivery. Each module type offers distinct advantages in terms of specificity, stability, and ease of conjugation to our various delivery systems.
Here are various ligand types used in B cell targeting:
| Ligand Type | Mechanism of Action | Targeted Marker(s) | Advantages/Application |
|---|---|---|---|
| Antibodies | Specialized proteins that recognize and attach to distinct B cell surface antigens. This attachment can directly influence B cell activity (e.g., stimulating, suppressing, eliminating) or act as a precise tether for therapeutic delivery platforms. |
|
Boasting remarkable selectivity and proven developmental paths, antibodies enable potent, lasting targeting. This makes them perfect for sustained therapeutic interventions and diagnostic visualization of B cell-associated conditions. |
| Peptide | Compact chains of amino acids crafted to interact with particular B cell receptors or surface components. Their reduced dimensions facilitate superior tissue permeation compared to larger antibody molecules. |
|
Characterized by their compact nature, effective tissue entry, extensive adaptability, and economical production, peptides can be designed to target distinct B cell activation profiles or subsets, providing precise regulatory command over B cell activity. |
| Carbohydrates | Sugar-based molecules (glycans) that engage with lectins, which are carbohydrate-binding proteins present on B cell surfaces, thereby enabling cell identification or internalization. |
|
Possessing inherent recognition pathways and potentially reduced immune reactivity, carbohydrate ligands can be employed to target B cells via specific lectin engagements, presenting an innovative approach to influencing B cell behavior. |
| Aptamers | Single-strand DNA or RNA sequences that assume distinct three-dimensional configurations, enabling strong and selective binding to particular B cell molecular targets, akin to antibody interactions. |
|
Exhibiting elevated specificity, compact dimensions, straightforward chemical synthesis and alteration, minimal immunogenicity, and robust stability, aptamers present an adaptable framework for B cell targeting, apt for both therapeutic and diagnostic uses. |
| Other | This group comprises diverse molecules, such as small compounds, signaling proteins (cytokines), or modified proteins, all engineered to engage with particular B cell receptors or cellular pathways. |
|
Offers the adaptability to investigate innovative targeting methodologies beyond conventional antibody or peptide strategies, potentially yielding groundbreaking B cell modulation techniques. |
Contact Us About B cell Targeting Module
What We Can Offer?
Creative Biolabs pioneers precision therapeutic delivery advancements, focusing expertise on B-cell engagement. Our expert team offers over two decades of experience in sophisticated delivery solutions, including:
Ready-to-Use Products
Customized Services
We develop tailored delivery systems and novel targeted modules from concept to validation, precisely meeting your project's unique specifications. This includes custom aptamer, peptide, or antibody synthesis/conjugation and optimization for specific B cell subsets.
Conjugation Services
Expert conjugation of ligands to various delivery platforms (nanoparticles, liposomes, polymers, etc.), ensuring optimal B cell targeting binding and stability.
Pre-Clinical Validation
Rigorous in vitro and in vivo testing to assess targeting efficiency, uptake, biodistribution, and therapeutic efficacy of B cell-targeted constructs.
Comprehensive Scientific Support
Leverage our deep scientific knowledge, state-of-the-art facilities, and rigorous quality control for your B cell targeting projects, from experimental design to data analysis.
Workflow

Why Choose Us?
Partnering with Creative Biolabs accelerates drug development, enhances therapeutic efficacy, and significantly reduces off-target effects for B cell-related therapies. Our commitment to innovation and scientific excellence ensures your therapeutic agents precisely reach B cell targets, unlocking new possibilities.
Proven Expertise
Our expert biologists, chemists, and engineers bring extensive scientific understanding to drug delivery systems and B cell targeting.
Innovative Technology
We employ cutting-edge technological platforms for module synthesis, precise conjugation, and thorough characterization, ensuring paramount quality and B cell targeting specificity
Tailored Customization & Flexibility
Our services include custom antibody, peptide, or aptamer design and delivery system optimization, precisely matching your therapeutic goals and target B cell populations.
Rigorous Quality & Reliability
Our steadfast commitment to scientific rigor consistently delivers dependable, reproducible, and high-quality results for your critical projects.
Published Data
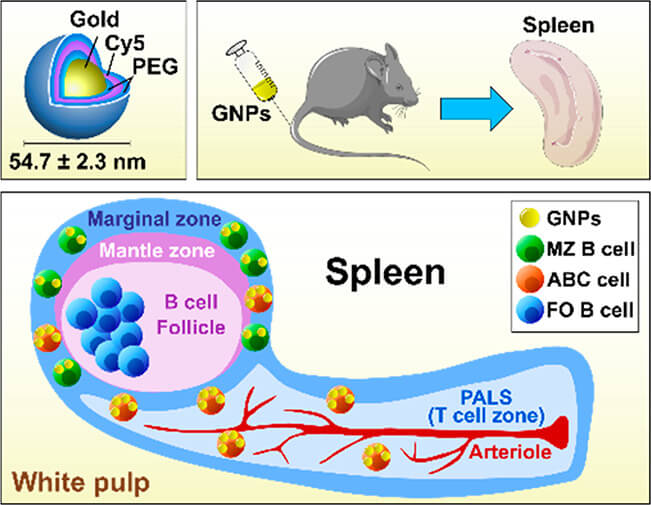 Fig.3 PEGylated Gold Nanoparticles Target Age-Associated B Cells in vivo.2,3
Fig.3 PEGylated Gold Nanoparticles Target Age-Associated B Cells in vivo.2,3
In the experiments, researchers synthesized fluorescently labeled, double PEGylated gold nanospheres, confirming their high colloidal stability in various biological media, which is crucial for consistent in vivo performance. Upon intravenous injection into mice, these GNPs primarily interacted with age-associated B cells (ABCs) and marginal zone (MZ) B cells within the spleen. This specific targeting is notable given the distinct roles these B cell subsets play in immune regulation. Crucially, the study found that the GNPs did not adversely affect the viability or proportions of B cells, nor did they induce undesirable innate-like immune responses or impair adaptive B cell responses in immunized mice. These findings highlight the safety profile and precise targeting capability of these polymer-coated GNPs for ABCs in vivo, suggesting their significant potential for targeted clinical applications, such as in autoimmune diseases or chronic infections, without broadly compromising overall B cell immune function.
FAQs
Q: How can targeted B cell delivery improve therapeutic outcomes?
A: Targeted delivery significantly enhances therapeutic outcomes by concentrating the drug payload directly at the site of disease-causing B cells. This precision minimizes exposure to healthy tissues, reducing systemic toxicity and allowing for higher, more effective doses to be delivered specifically to the target cells, ultimately improving efficacy and patient safety.
Q: Can you target specific B cell subpopulations (e.g., plasma cells vs. memory B cells)?
A: Yes, absolutely. Our expertise extends to developing highly specific targeting modules that differentiate between various B cell subpopulations. By leveraging distinct surface markers and receptor expression profiles, we can design solutions for highly nuanced and precise therapeutic interventions tailored to specific disease states.
Q: What types of B cell-targeting ligands can you provide?
A: We offer a diverse range of targeting ligands, including but not limited to: peptides, monoclonal antibodies and their fragments (e.g., Fab, scFv), aptamers, and carbohydrate-based ligands. Our team can help you select or design the most appropriate ligand for your specific B cell target.
Q: What are the typical applications for B cell targeting modules?
A: B cell targeting modules have broad applications in immunology and oncology. They are crucial for developing advanced therapies for autoimmune diseases (e.g., lupus, rheumatoid arthritis), B cell lymphomas and leukemias, and for enhancing vaccine efficacy by precisely modulating B cell responses. They can also be used for diagnostic imaging of B cell-related conditions.
Q: Can your B cell targeting modules be integrated with various drug delivery systems?
A: Absolutely. Our module design considers compatibility with various drug delivery platforms from the outset. Whether it's liposomes, LNPs, polymer nanoparticles, or others, we can provide coupling strategies and technical support to equip your chosen delivery vehicle with targeting capabilities, aiming for enhanced drug delivery efficiency and specificity.
Contact Our Team for More Information and to Discuss Your Project.
References
- Robinson, William H et al. "Cutting-edge approaches to B-cell depletion in autoimmune diseases." Frontiers in immunology vol. 15 1454747. 9 Oct. 2024, DOI:10.3389/fimmu.2024.1454747.
- Hočevar, Sandra et al. "PEGylated Gold Nanoparticles Target Age-Associated B Cells In Vivo." ACS nano vol. 16,11 (2022): 18119-18132. DOI:10.1021/acsnano.2c04871.
- Distributed under Open Access license CC BY 4.0, without modification.



