
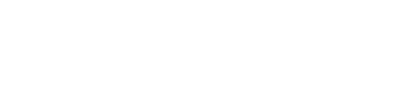
-
Solutions
- Subcellular Organelle Targeting Module Development
- Mitochondria Targeting Module Development
- Nucleus Targeting Module Development
- Cytosol Targeting Module Development
- Lysosome Targeting Module Development
- Golgi Targeting Module Development
Creative Biolabs enables precise drug delivery to subcellular organelles with our advanced targeting module platforms.
- Cell Targeting Module Development
- Neuron Targeting Module Development
- Microglia Targeting Module Development
- Astrocyte Targeting Module Development
- T Cell Targeting Module Development
- B Cell Targeting Module Development
- Macrophage Targeting Module Development
- NK Cell Targeting Module Development
- Neutrophil Targeting Module Development
- Endothelium Targeting Module Development
- Cancer Cell Targeting Module Development
Creative Biolabs develops cell-specific targeting modules to enhance therapeutic accuracy and efficacy.
- Microorganism Targeting Module Development
Creative Biolabs designs microorganism-targeting modules for precise antimicrobial and diagnostic applications.
- Disease Targeting Module Development
- Lymphoma Targeting Module Development
- Fibrosarcoma Targeting Module Development
- Glioma Targeting Module Development
- Hepatocellular Carcinoma Targeting Module Development
- Hepatitis Targeting Module Development
- Atherosclerosis Targeting Module Development
- Bladder Cancer Targeting Module Development
- Esophageal Cancer Targeting Module Development
- Alzheimer's Disease Targeting Module Development
- Hepatic Fibrosis Targeting Module Development
- Kidney Fibrosis Targeting Module Development
- Pulmonary Arterial Hypertension Targeting Module Development
- Osteosarcoma Targeting Module Development
- Lung Adenocarcinoma Targeting Module Development
- Diabetes Targeting Module Development
- Cervical Cancer Targeting Module Development
Creative Biolabs designs disease-specific targeting modules to improve drug delivery and therapeutic outcomes.
- Organ/Tissue Targeting Module Development
- Heart Targeting Module Development
- Liver Targeting Module Development
- Spleen Targeting Module Development
- Lung Targeting Module Development
- Kidney Targeting Module Development
- Bone Targeting Module Development
- Brain Targeting Module Development
- Prostate Targeting Module Development
- Adipose Tissue Targeting Module Development
- Eye Targeting Module Development
- Joint Targeting Module Development
Creative Biolabs creates organ- and tissue-targeting modules for site-specific drug delivery and enhanced efficacy.
- Subcellular Organelle Targeting Module Development
-
Services
- Module Delivery Systems
- Liposome
- LNP
- Exosome
- VLP
- Micelles
- Hydrogels
- Fc Fusion Protein
- Albumin
- CPP
- Graphene Oxide
- Hyaluronic Acid
- Elastin-like Polypeptide
- Polymeric Microsphere
- Electrospun Polymeric Nanofiber
Creative Biolabs provides tailored delivery system development to optimize the transport and release of therapeutic agents.
- Targeted Module & Chemical Synthesis
Creative Biolabs offers customized synthesis of targeting modules and chemical compounds for advanced drug delivery solutions.
- Bioconjugate
Creative Biolabs specializes in bioconjugation to link targeting modules with therapeutic payloads or delivery system for enhanced delivery.
- Characterization

Creative Biolabs specializes in the thorough characterization of targeted delivery systems, delivering complete insights to advance your research.
- Module Delivery Systems
- Products
-
Platform
- SORT LNP Platform
Creative Biolabs' SORT LNP platform enables precision organ targeting, developing next-generation lipid nanoparticles for highly specific therapeutic delivery.
- Drug Delivery Platform

For versatile small molecule delivery, Creative Biolabs offers a platform to engineer module delivery systems for targeted applications.
- In Vitro Assay Platform

Accelerate your research with Creative Biolabs' in vitro platform, which develops custom assays to characterize targeting and efficacy.
- In Vivo Assay Platform
Advance your therapeutic candidates with critical in vivo data from Creative Biolabs' PK/PD and biodistribution study platform.
- Manufacturing Platform

Creative Biolabs provides reliable, large-scale manufacturing of targeted delivery systems under strict quality control, ensuring consistency for your programs.
- Bioconjugation Platform
Bioconjugation is key to targeted delivery, and Creative Biolabs' platform provides expert services to create advanced conjugates.
- SORT LNP Platform
- Support & Resources
- Company


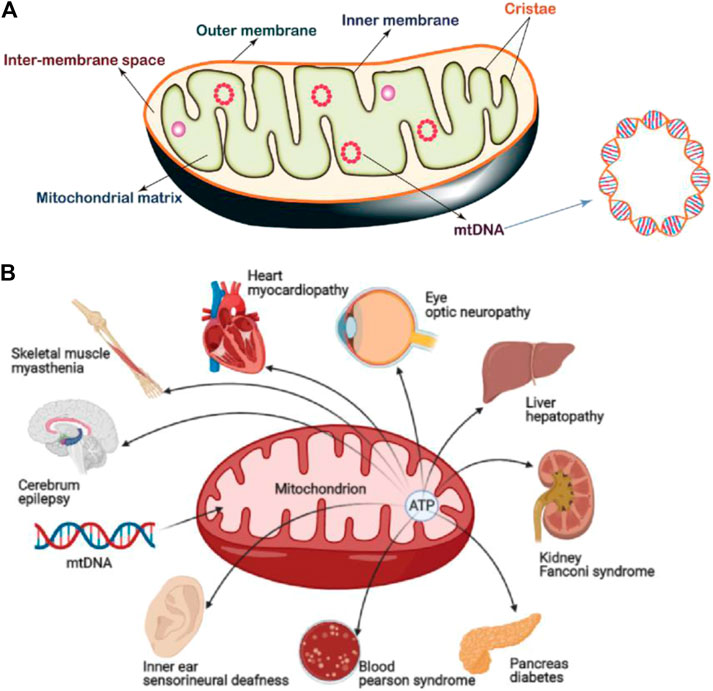 Fig 1. Mitochondrial structure and mitochondrial diseases.1,3
Fig 1. Mitochondrial structure and mitochondrial diseases.1,3
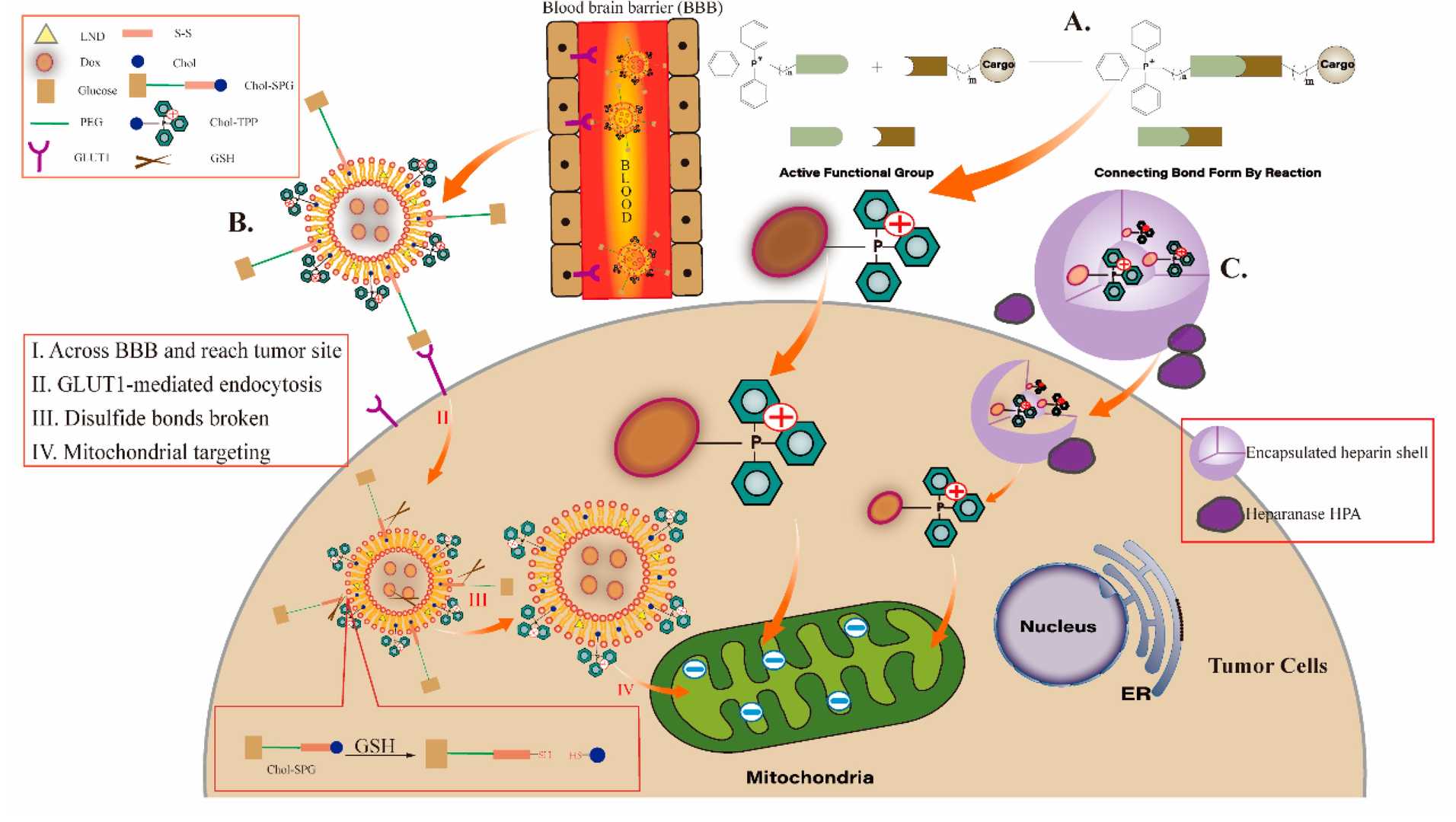 Fig.2 TPP-based mitochondria-targeted anticancer drugs.2,3
Fig.2 TPP-based mitochondria-targeted anticancer drugs.2,3
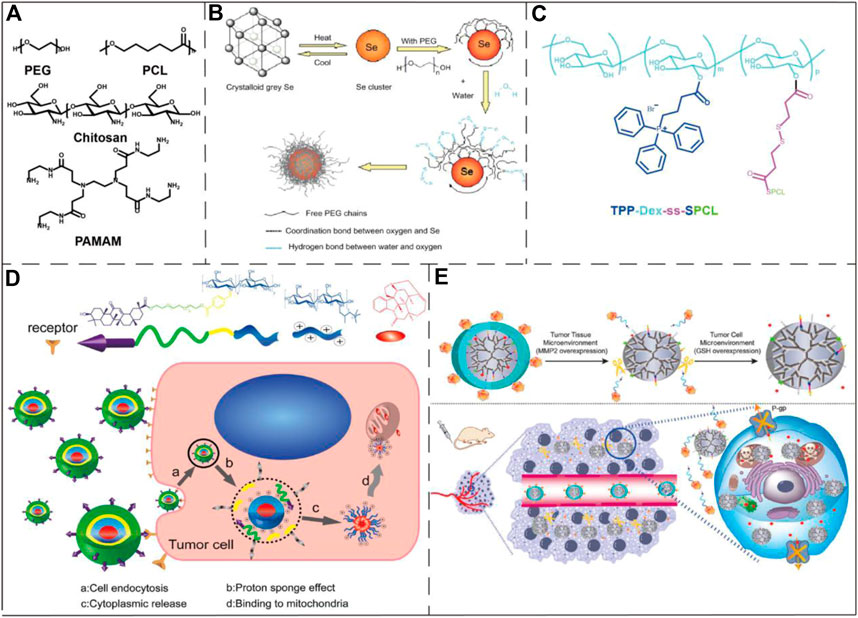 Fig 3. Examples of polymeric/polymer-coated nanoparticles and micelles in the application of drug delivery to mitochondria.1,3
Fig 3. Examples of polymeric/polymer-coated nanoparticles and micelles in the application of drug delivery to mitochondria.1,3


