Targeted Delivery System Characterization Service
Developing a safe and effective therapeutic hinges on the precision of its delivery system. At Creative Biolabs, we provide the essential, high-resolution characterization services that validate your targeted delivery systems—from lipid-based drug delivery systems to exosomes. Our expertise ensures you can confidently navigate the complexities of formulation development, secure in the knowledge that your payload will be delivered effectively and safely.
Overcoming the Delivery Challenge: Stability and Biological Barriers
The therapeutic index of a drug is profoundly enhanced by its ability to reach the intended site of action. This is particularly true for complex payloads like nucleic acids and protein degraders, which are fragile and require protection from biological barriers (like cell membranes and enzymatic degradation). Comprehensive characterization provides the critical data to confirm that your delivery system is structurally sound, stable, and capable of specific cellular targeting, thereby maximizing therapeutic efficacy and minimizing off-target toxicity.
The Analytical Triad: Our Comprehensive Validation Approach
Successful drug delivery requires data across three core dimensions. Creative Biolabs' services are designed to address each pillar, ensuring orthogonal validation of your therapeutic candidate:
- Physicochemical Attributes: Focuses on the physical identity of the particle, including size, size distribution, morphology, and surface charge (Zeta Potential). This data is essential for predicting biodistribution and colloidal stability.
- Payload and Purity: Measures the quantity and integrity of the therapeutic cargo (Drug Loading, Encapsulation Efficiency), as well as the purity of the delivery vehicle components. This validates the dosage and chemical stability.
- Performance and Stability: Predicts how the system will function over time and in vivo through assays like stability testing (under various stress conditions) and in vitro release kinetics.
Our Comprehensive Characterization Services for Targeted Delivery
At Creative Biolabs, we understand that robust characterization is the bedrock of successful drug delivery. Our characterization services are designed to provide the critical, high-resolution data you need to validate your formulations, optimize performance, and move your research forward with confidence. Our capabilities span foundational physicochemical analysis to advanced biological assessments, ensuring every aspect of your formulation is thoroughly evaluated.
Particle Size & Distribution
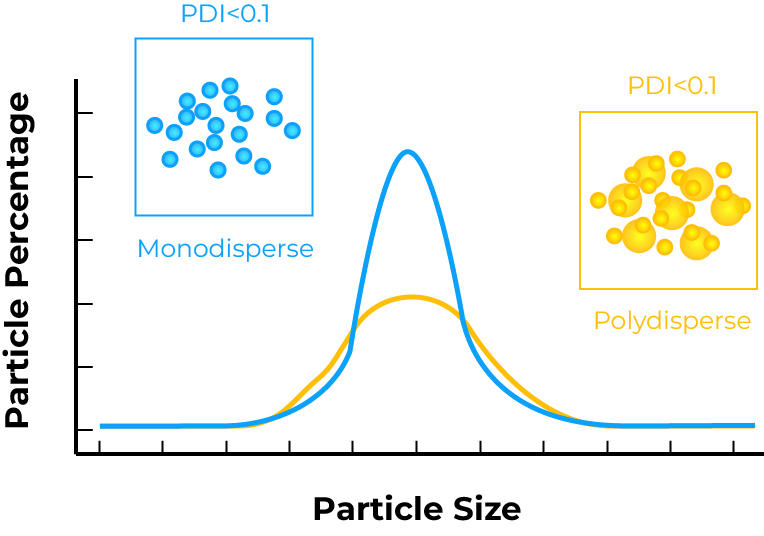
The size and size distribution of a delivery system are fundamental to its function, influencing everything from cellular uptake and biodistribution to colloidal stability and therapeutic efficacy. A tight and reproducible size distribution (low PDI) is a critical quality attribute (CQA) for any advanced therapeutic.
Analytical Methods
We employ a suite of state-of-the-art technologies to precisely measure particle size and concentration.
- Dynamic Light Scattering (DLS)
- Nanoparticle Tracking Analysis (NTA)
- Atomic Force Microscopy (AFM)
We aim for a polydispersity index (PDI) of less than 0.3, a common standard for demonstrating a narrow size distribution and formulation homogeneity.
Shape & Morphology

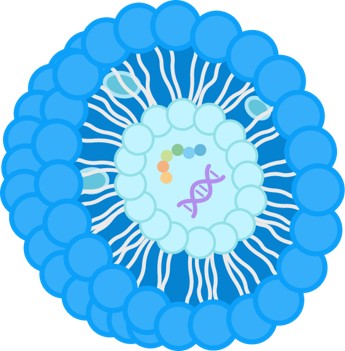
Understanding the physical appearance and structure of your nanoparticles is crucial for assessing their quality and predicting their performance in vivo. We provide detailed analysis to confirm the integrity of your delivery system and identify potential issues like aggregation or defects.
Analytical Methods
We utilize advanced microscopy techniques to capture high-resolution images of your particles.
- Scanning Electron Microscope (SEM)
- Transmission Electron Microscopy (TEM)
- Cryo-Electron Microscopy (Cryo-EM)
Our high-resolution imaging protocols deliver precise imaging of nanoparticles like liposome, LNPs and exosomes, enabling accurate analysis of morphology and size distribution to support formulation development and troubleshooting.
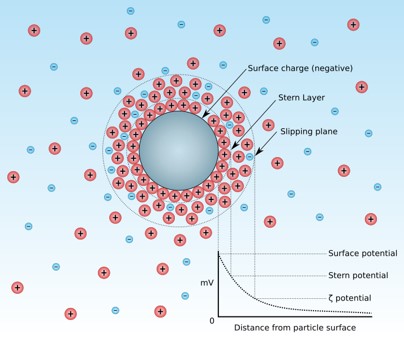
Zeta Potential

 Distributed under CC BY-SA 3.0, from Wiki, without modification.
Distributed under CC BY-SA 3.0, from Wiki, without modification.
Zeta potential, a measure of the surface charge of your particles, is a key indicator of colloidal stability. A high zeta potential—either positive or negative—suggests that particles will repel each other, preventing aggregation and extending the shelf life of your formulation.
Analytical Methods
Our team uses industry-leading instruments to accurately measure the surface charge of your particles, a key parameter for predicting colloidal stability and preventing unwanted aggregation.
- Electrophoretic Light Scattering (ELS)
- Zeta Potential Analyzer
We follow established protocols for sample preparation and measurement to ensure reproducible and reliable zeta potential data.
Structure & Composition

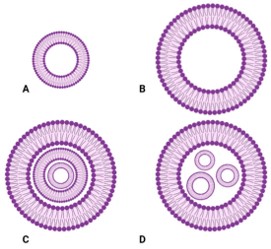
The crystalline structure and composition of your formulation materials directly impact the stability and release profile of your drug. Our services provide a precise analysis to ensure the physical state of your drug product is consistent and meets quality standards.
Analytical Methods
We provide comprehensive analysis of the physical state and chemical makeup of your formulation. This is crucial for verifying the consistency of your material from batch to batch.
- X-ray Diffraction (XRD)
- Differential Scanning Calorimetry (DSC)
- Fourier-Transform Infrared Spectroscopy (FTIR)
We have extensive experience analyzing the complex crystalline structures, providing critical data for the selection of optimal excipients and formulation processes.
Drug Loading & Encapsulation Efficiency
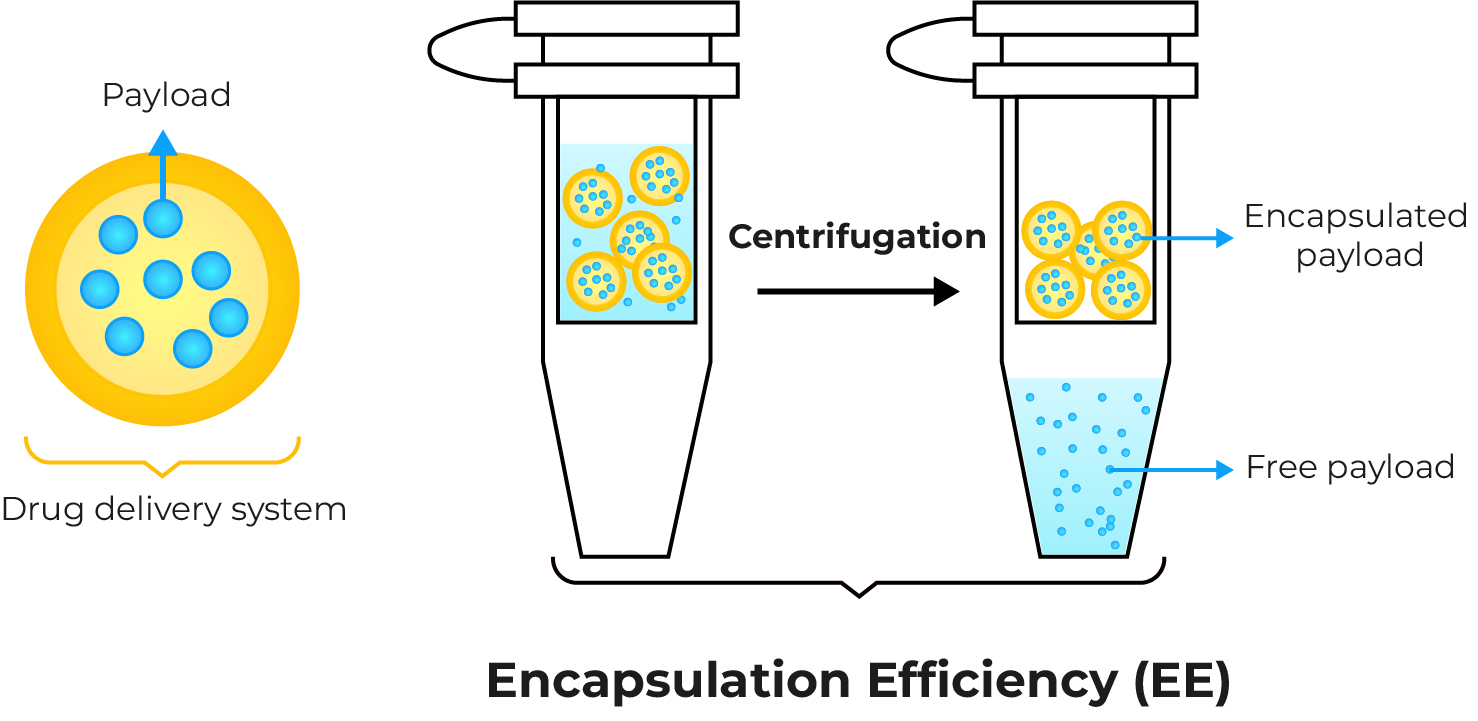
These metrics quantify how much of your therapeutic payload is successfully encapsulated. We develop and validate robust analytical methods to provide precise measurements, which are fundamental for optimizing your formulation and ensuring consistent dosage.
Analytical Methods
We employ a variety of high-performance analytical techniques to provide accurate and reliable data on your drug loading (DL) and encapsulation efficiency (EE).
- High-Performance Liquid Chromatography (HPLC)
- UV-Vis Spectrophotometry
- Bicinchoninic Acid (BCA) Assay
- RiboGreen Assay
We offer the quantitative analysis for each type of payload to perform the DL and EE calculations. If necessary, we can also undertake the development of separate quantitative methodologies.
Stability Analysis

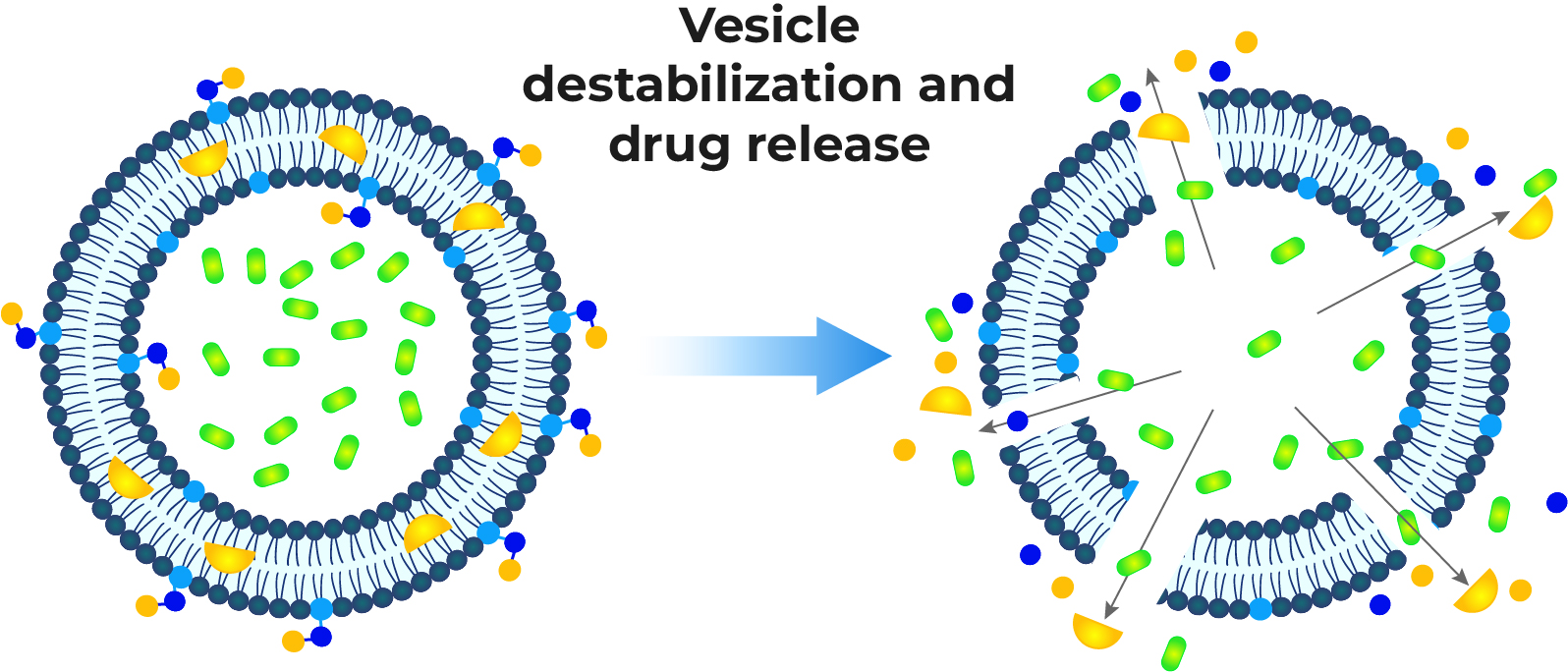
We conduct comprehensive stability studies to evaluate the physical, chemical, and biological integrity of your formulation over time. This includes assessing the impact of various stress conditions, which is essential for determining shelf life and ensuring product quality.
Analytical Methods
Our stability analysis is multi-faceted, utilizing a range of techniques to monitor the physical and chemical changes in your formulation over time.
- DLS for size stability
- TEM for morphological stability
- HPLC for chemical stability
- Circular Dichroism (CD) for protein structural stability
We offer a comprehensive, integrated approach to stability, combining multiple analytical techniques to track physical, chemical, and biological stability in parallel, providing a holistic view of your formulation's long-term performance.
In Vitro Release Testing
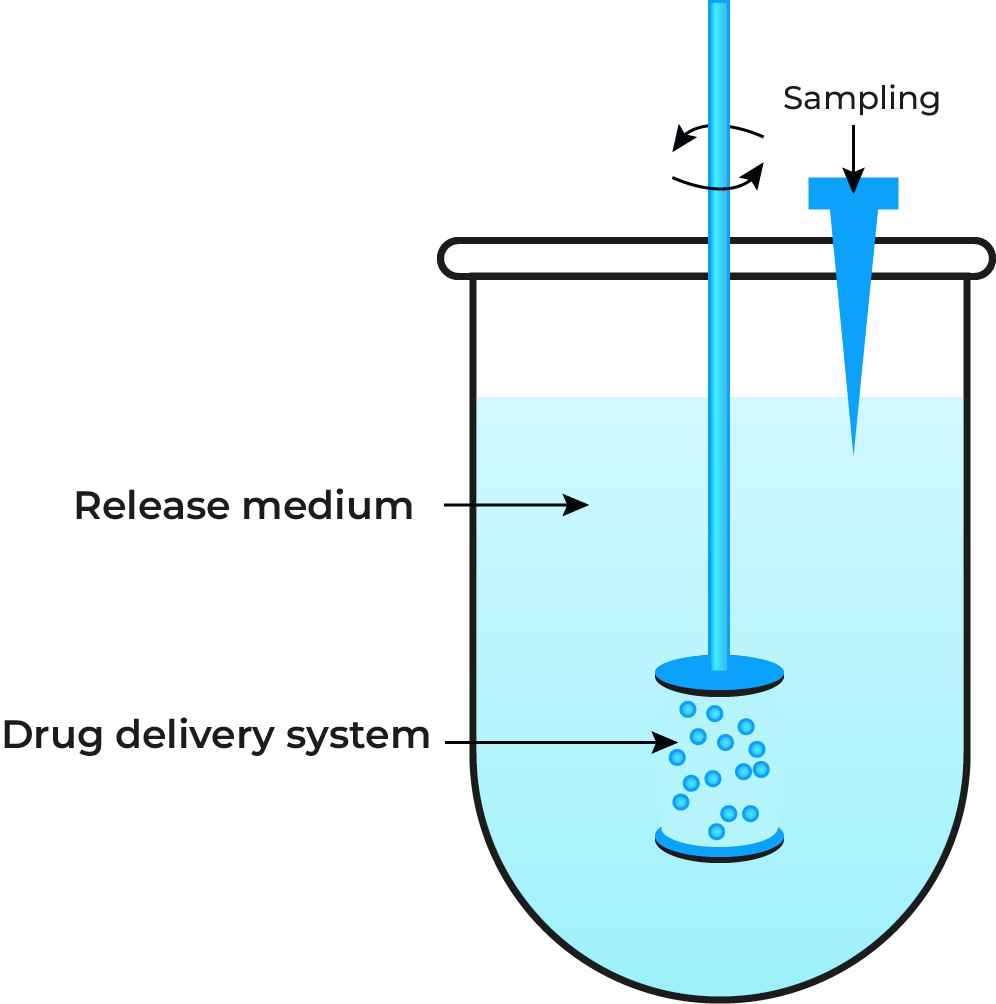
To be effective, a drug must be released from its delivery system at the right time and place. In vitro release testing is a predictive tool that models this process under simulated biological conditions. It provides crucial insight into the mechanism and duration of drug release.
Analytical Methods
Our ability to customize release conditions to your specific application—for example, by varying pH, temperature, or the presence of enzymes—allows us to accurately model the unique conditions of your targeted site.
- Dialysis-Based Methods
- Quantitative Payload Analysis
We have a rich history of developing and executing in vitro release assays for a diverse range of payloads, ensuring we can handle your most challenging projects.
Workflow

Case Studies
Case Study 1: High-Resolution TEM Analysis of Conventional Liposomes

The TEM analysis confirmed the liposomes were uniformly distributed and spherical, with a highly consistent particle size of 100 nm. This provided the necessary quality control evidence for advancement.
- Morphology: Uniformly distributed and spherical.
- Size Consistency: Highly consistent.
- Size: ~100 nm
Case Study 2: Quantitative EE Analysis of Drug-Loaded Liposomes
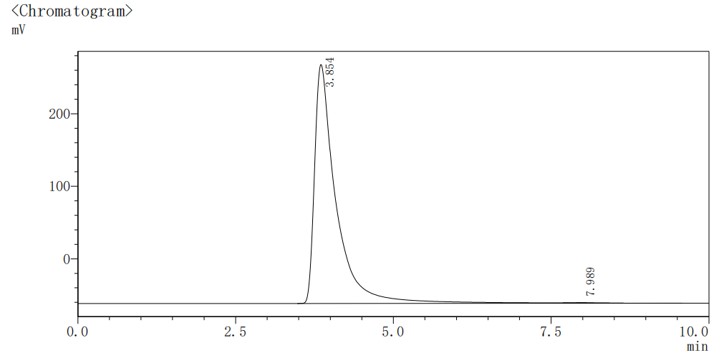
We successfully established a robust HPLC quantitative method tailored specifically for the drug, allowing for precise measurement of both free and encapsulated drug concentrations. This validated analytical method enabled the accurate calculation of DL and EE, providing the client with critical data to optimize their formulation.
- Method: Validated HPLC
- Quantifies: Free & encapsulated drug
- Outcome: Accurate DL & EE values
Case Study 3: Comprehensive Characterization of mRNA-LNP
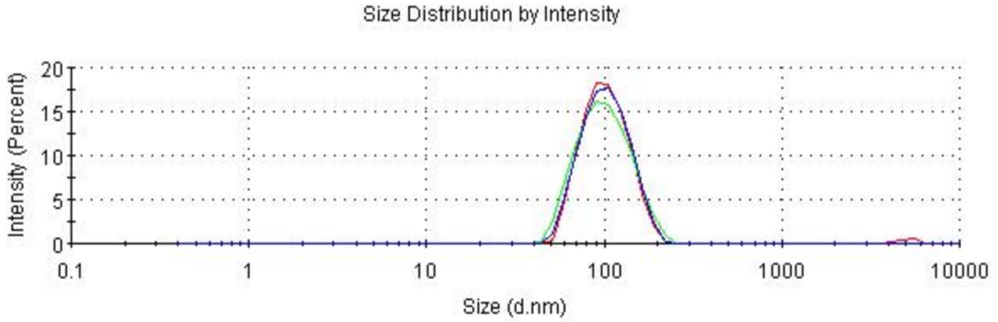
We have comprehensively characterized the customized mRNA-LNP using DLS for particle sizing and the RiboGreen assay for quantifying nucleic acid encapsulation, covering the core quality attributes.
- Size: ~100 nm
- Polydispersity Index (PDI): ~0.15
- Zeta Potential: −5 mV
- Encapsulation Efficiency (EE): >99%
Applications of Characterization in Modern Therapeutic Research
Rigorous characterization is the foundational necessity across the modern therapeutic landscape. It is not merely a quality check but a necessary step that enables successful drug development. The following fields rely heavily on robust characterization data to ensure the performance and safety of diverse therapeutic modalities:
- Vaccine Development: Crucial for the development of mRNA and DNA vaccines, ensuring the stability and integrity of the nucleic acid-LNP complex.
- Gene & Cell Therapy: Essential for validating the delivery systems used to introduce CRISPR-Cas9 components, plasmids, or siRNA into target cells.
- Oncology: Provides critical data for optimizing nanoparticle formulations for targeted cancer therapy, ensuring drugs reach tumor cells while minimizing systemic toxicity.
- Autoimmune & Inflammatory Diseases: Enables the development of targeted delivery systems for immunomodulatory drugs, reducing off-target effects and improving patient outcomes.
Why Choose Creative Biolabs?
When you choose Creative Biolabs, you gain a partner dedicated to your success, offering a unique combination of scientific excellence and client-centric collaboration.
Two Decades of Expertise
Our team of PhD-level scientists brings extensive, hands-on experience in drug delivery and formulation science.
Authority in Advanced Technologies
We are pioneers in the application of Cryo-EM and other high-end analytical tools for complex delivery systems.
Tailored, Flexible Solutions
We offer a blend of ready-to-use products and customized services that are perfectly aligned with your unique research needs.
Accelerated Innovation
Our efficient workflows and rapid turnaround times help your de-risk projects and expedite your path to groundbreaking discoveries.
Creative Biolabs is your trusted partner for rigorous and comprehensive delivery system characterization. Our full suite of representation services covers every critical quality attribute, from precision particle sizing (DLS, NTA) and structural validation (TEM, Cryo-EM) to accurate payload quantification (HPLC) and predictive stability testing (ICH). Don't let characterization be a bottleneck—contact us today to discuss your project and secure the foundational data for your therapeutic success.
Related Services
Related Products
| Product Name | Description | Inquiry |
|---|---|---|
| RNA-LNP Encapsulation Assay Kit | A validated, quantitative kit for rapidly measuring the encapsulation efficiency of mRNA or siRNA in LNPs. | |
| LNP Formulation Kit (Universal) | A core kit providing essential lipids and buffers for basic, highly efficient LNP self-assembly. |
FAQs
What are the Critical Quality Attributes (CQAs) of a delivery system that Creative Biolabs characterizes?
The primary CQAs we focus on include particle size and PDI, zeta potential, DL, and EE. These core attributes are directly linked to the therapeutic product's fundamental performance and safety. Additionally, more complex analyses, such as physical and chemical stability testing and morphology (shape/integrity) analysis, are available as supplementary characterization items.
What is the expected PDI for advanced nanoparticle formulations like LNPs?
While specific requirements vary by application, we generally aim for a PDI of <0.3 for monodisperse delivery systems. A PDI below this threshold typically indicates a tight, uniform distribution essential for predictable in vivo behavior and high colloidal stability.
Can you perform characterization experiments under simulated in vivo conditions?
Yes. We frequently customize our assays, particularly for stability analysis and in vitro release testing, to mimic relevant biological conditions such as varying pH (e.g., endosomal pH), temperature, and the presence of biological components like serum or specific enzymes. This provides data highly predictive of in vivo performance.
What is the minimum sample volume required for your characterization services?
The minimum sample volume varies depending on the specific assays requested. Please refer to our detailed service specifications or consult with our technical support team for precise requirements tailored to your project.
Can you provide customized assays for unique delivery systems?
Yes, our scientific team specializes in developing and validating custom analytical methods for novel or unconventional drug delivery systems. We work closely with our clients to design a characterization plan that meets their specific needs.
What is the typical turnaround time for a complete characterization project?
The project timeline depends on the complexity and number of assays required. Following our initial project scoping, we provide a detailed timeline, with most projects ranging from 2-6 weeks from sample submission to final report delivery.
References
- Ferraro, Claudia, et al. "Exploring protein-based carriers in drug delivery: a review." Pharmaceutics 16.9 (2024): 1172. https://doi.org/10.3390/pharmaceutics16091172.
- Strazzabosco, Giovanni, et al. "Insights into Liposomal and Gel-Based Formulations for Dermatological Treatments." Gels 11.4 (2025): 245. https://doi.org/10.3390/gels11040245
- Distributed under an Open Access license CC BY 4.0, without modification.