We offer both in vitro and in vivo experimental platforms to support the development and validation of targeted drug delivery systems. Our capabilities allow us to track your drug from the cellular level to a whole-organism model.
In Vivo PK/PD & Biodistribution Study Platform
Are you currently facing complex clinical trials, long drug development cycles, or challenges in targeted drug delivery? Creative Biolabs' In Vivo PK/PD & Biodistribution Studies help you accelerate drug discovery by providing a deep understanding of your compound's behavior in a biological system. We achieve this through advanced in vitro and in vivo experimental platforms, delivering a comprehensive analysis of your therapeutic agent's safety and efficacy profile.
In Vivo PKPD and Biodistribution
The journey of a therapeutic agent, from administration to effect, is a complex process. Pharmacokinetics (PK) describes how the body affects a drug, including its absorption, distribution, metabolism, and excretion. Pharmacodynamics (PD), on the other hand, describes how the drug affects the body. Biodistribution is a critical component of the PK profile, specifically focusing on the spatial and temporal distribution of a compound within a biological system. The analysis of these profiles is indispensable for modern drug development. It provides the foundational data required to predict a drug's safety, determine its effective dosage, and confirm that it reaches its intended biological target. A deep understanding of a drug's PK/PD and biodistribution is a prerequisite for successful clinical translation and regulatory approval.
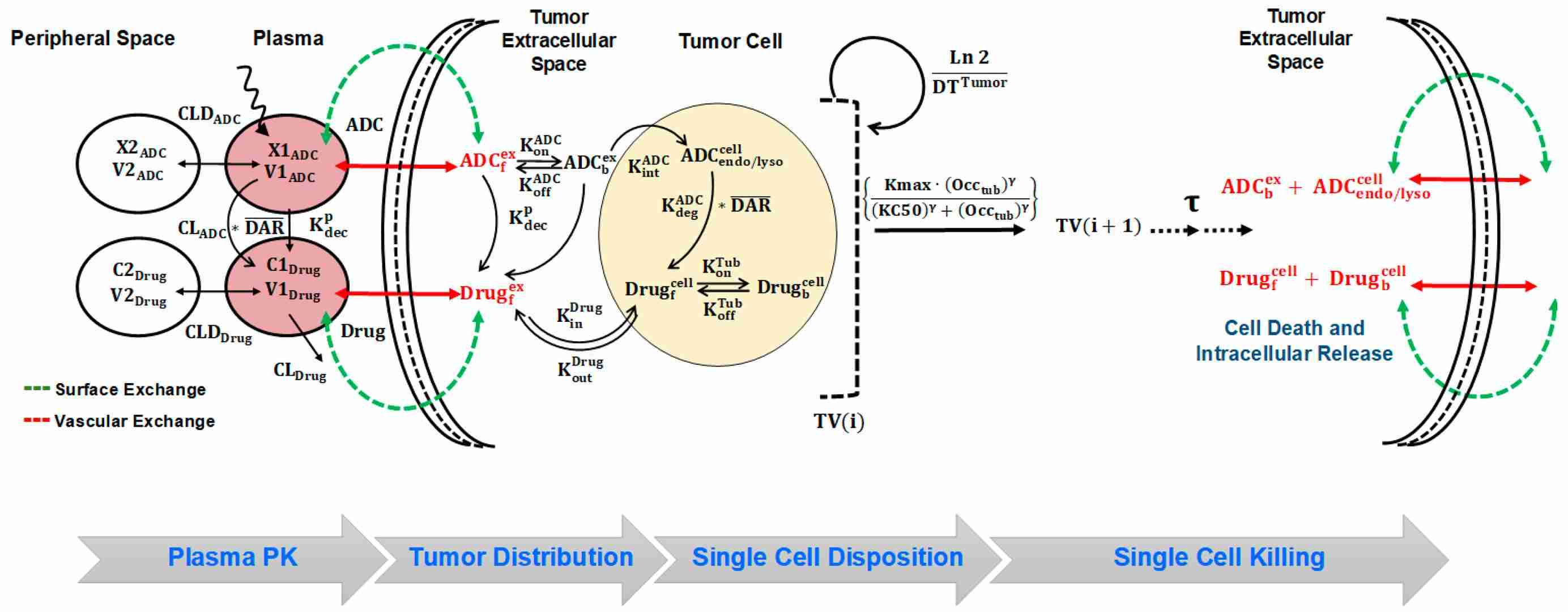 Fig.1 The systems pharmacokinetics-pharmacodynamics (PK-PD) model for antibody-drug conjugates (ADCs).1,3
Fig.1 The systems pharmacokinetics-pharmacodynamics (PK-PD) model for antibody-drug conjugates (ADCs).1,3
Our In Vivo Solution
Creative Biolabs offers an integrated, end-to-end solution for a comprehensive analysis of your compound's PK/PD and biodistribution profiles. Our approach combines state-of-the-art platforms with expert analysis to provide you with the critical data needed for informed decisions.
- Integrated PK/PD and Biodistribution Analysis: We provide a unified approach to analyzing all aspects of your compound's behavior in vivo. Our studies provide quantitative data on key pharmacokinetic parameters such as Cmax, AUC, and half-life (t1/2n). We correlate these with pharmacodynamic endpoints to establish a clear relationship between drug exposure and therapeutic effect. For biodistribution, we meticulously measure the concentration of your compound in target and non-target tissues over time, providing a detailed map of its fate.
- Validation of Advanced Drug Delivery Systems: Our platforms are specifically designed to evaluate innovative drug delivery systems, including liposomes, exosomes, and nanoparticles. We assess how these systems improve drug properties, such as stability and solubility, and evaluate their ability to achieve targeted delivery, reduce off-target effects, and enhance the drug's overall bioavailability and efficacy.
- Customizable and Comprehensive Study Design: We offer unparalleled flexibility in designing studies that meet your project's unique requirements. Our extensive portfolio includes a variety of animal models (e.g., mice, rats, non-human primates) and cell models, as well as proficiency in numerous routes of administration. We tailor each study to provide the most relevant and robust data for your specific therapeutic agent.
Application
Creative Biolabs offers an integrated, end-to-end solution for a comprehensive analysis of your compound's PK/PD and biodistribution profiles. Our approach combines state-of-the-art platforms with expert analysis to provide you with the critical data needed for informed decisions.
In vivoPK/PD and biodistribution studies are essential for the development of targeted therapies across various fields:
- Oncology: These studies confirm that drug-carrying nanoparticles successfully target tumors, minimizing harm to healthy tissue.
- Gene Therapies: They track the distribution of viral vectors to ensure they reach the correct tissue and avoid accumulating in non-target organs.
- Immunology: The studies validate that immunomodulatory drugs successfully reach specific immune cells to achieve the desired effect.
- Infectious Diseases: These studies are used to confirm that antimicrobial agents or vaccines delivered via targeted systems reach the site of infection with high precision, improving treatment outcomes.
- Neuroscience: They are vital for evaluating whether therapeutic agents can successfully cross the blood-brain barrier and accumulate in specific brain regions to treat neurological disorders.
Our solutions provide the critical data needed to drive innovation in these areas.
Contact Us About Bioconjugation Services
What We Can Offer?
Creative Biolabs is uniquely positioned at the forefront of targeted drug delivery and preclinical research. We provide comprehensive solutions to validate the efficacy and safety of your therapeutic agents.
Integrated Platforms
Enhanced Drug Delivery
We can help you design and evaluate drug delivery systems that improve your drug's physicochemical properties and enhance targeted delivery. This directly impacts pharmacokinetics, leading to enhanced bioavailability and efficacy.
Published Data
Our methodologies are supported by a strong foundation of scientific rigor, including published data that demonstrates the successful application of our platforms in complex therapeutic areas.
Diverse Models and Administration
We provide a wide range of animal and cell models to match your specific research needs. We also support various routes of administration, including oral, intravenous, intraperitoneal, subcutaneous, and intranasal.
Workflow

Why Choose Us?
Choosing Creative Biolabs means partnering with a team dedicated to innovation and scientific excellence, ensuring your therapeutic agents reach their targets with unprecedented precision.
Proven Expertise
Our team of highly specialized biologists, chemists, and engineers possesses over 20 years of collective experience and deep scientific knowledge in drug delivery systems and targeting module development.
Innovative Technology
We leverage state-of-the-art platforms for module synthesis, conjugation, and characterization, giving you a competitive edge in your research.
Tailored Customization & Flexibility
We offer customized aptamer/peptide design and delivery system optimization tailored to your specific therapeutic goals.
Rigorous Quality & Reliability
Our commitment to scientific rigor ensures reliable, reproducible, and high-quality results for your critical projects. Our internal quality control processes are meticulous, guaranteeing the integrity of your data.
Published Data
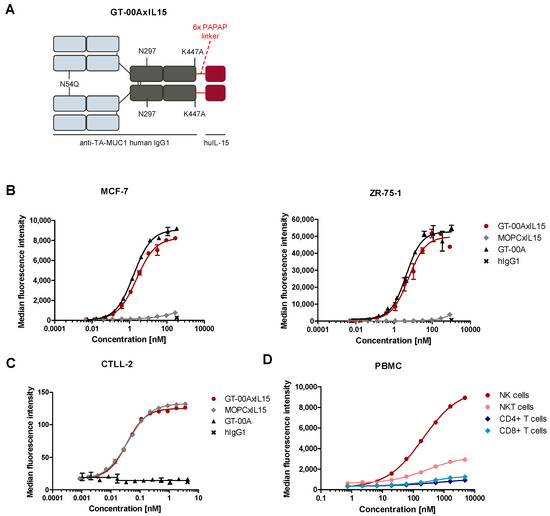 Fig.2 GT-00AxIL15 binds to its targets TA-MUC1 and IL-15R on human tumor cell lines and immune cells.2,3
Fig.2 GT-00AxIL15 binds to its targets TA-MUC1 and IL-15R on human tumor cell lines and immune cells.2,3
The preclinical evaluation of GT-00AxIL15 involved a comprehensive series of both in vitro and in vivo experiments to assess its potential as a therapeutic agent for cancer. The in vitro phase, which focused on controlled laboratory studies outside a living organism, concentrated on the foundational properties of the immunocytokine. This included not only its generation but also a crucial step of radiolabeling GT-00AxIL15 and a control substance with zirconium-89. The use of this radioactive isotope was essential for subsequent biodistribution analysis, as it allowed researchers to accurately track the compound's movement, accumulation, and clearance within living organisms.
Building upon these initial findings, the in vivo studies were designed to evaluate the compound's effects and distribution within living systems. A key component of this phase was a 4-week toxicology study conducted in rats. In this study, repeated intravenous doses of up to 2.5 mg/kg of GT-00AxIL15 were administered to thoroughly assess its safety profile. A significant and encouraging finding was the complete absence of any test item-related side effects or observable signs of inflammation, which is a critical indicator of a compound's tolerability. Furthermore, the immunocytokine successfully elicited the expected pharmacodynamic effects on key components of the immune system, specifically NK, NKT, and cytotoxic T cells in the blood. This activation of immune cells is a direct measure of the drug's intended function and confirms that it is successfully stimulating an anti-tumor immune response. These combined findings—demonstrating a favorable safety profile, effective targeting, and the desired immune activation—collectively provide a strong basis for the continued development of GT-00AxIL15 as a potential cancer therapeutic.
FAQs
Q: What factors influence the timeline and design of these studies?
A: The study timeline and design are highly customized based on your project's specific needs. Key factors include the therapeutic agent's complexity, the number of time points required for analysis, the chosen animal model, and the analytical methodologies, such as LC-MS/MS or immunoassays. We collaborate closely with you to create a scientifically robust and efficient study plan.
Q: What methodologies are used to analyze different types of therapeutic agents?
A: Our platforms are equipped to handle a wide range of compounds. We utilize specialized analytical techniques, such as LC-MS/MS for small molecules, ELISA and other immunoassays for biologics like antibodies, and qPCR or ddPCR for gene therapies, ensuring accurate quantification of your compound in biological matrices.
Q: How do PK/PD studies inform optimal dosage and administration?
A: Our studies provide crucial pharmacokinetic parameters, including Cmax, Tmax, and AUC, which define drug exposure. By correlating these with pharmacodynamic biomarkers, we can establish the therapeutic window. This integrated analysis is fundamental for selecting the most effective route of administration and optimizing the dose to achieve the desired therapeutic effect with minimal toxicity.
Q: What key data points and deliverables are included in a study report?
A: You will receive a comprehensive report that includes raw data sets, quantitative measurements of drug concentration in target and non-target tissues, graphical representations of concentration-time curves, and a detailed analysis of key PK parameters like half-life, clearance rate, and volume of distribution. Our reports are designed to provide clear, actionable insights.
Q: Why are in vivo studies indispensable, even with extensive in vitro data?
A: While in vitro data provides valuable mechanistic insights, it cannot fully replicate the complex interactions within a living organism. In vivo studies are essential for understanding systemic factors like metabolism, immune responses, and the full ADME profile, providing a more predictive and clinically relevant picture of a compound's behavior.
Ready to gain a deeper understanding of your therapeutic agent? Partner with Creative Biolabs to leverage our expertise in In vivo PK/PD & Biodistribution Studies.
Connect with our experts for project-specific consultation and detailed insights.
References
- Singh, Aman P et al. "A Cell-Level Systems PK-PD Model to Characterize In Vivo Efficacy of ADCs." Pharmaceutics vol. 11,2 98. 25 Feb. 2019, https://doi.org/10.3390/pharmaceutics11020098
- Gellert, Johanna et al. "GT-00AxIL15, a Novel Tumor-Targeted IL-15-Based Immunocytokine for the Treatment of TA-MUC1-Positive Solid Tumors: Preclinical In Vitro and In Vivo Pharmacodynamics and Biodistribution Studies." International journal of molecular sciences vol. 25,3 1406. 24 Jan. 2024, https://doi.org/10.3390/ijms25031406
- Distributed under Open Access license CC BY 4.0, without modification.



