Development of novel peptides, aptamers, or small molecules with high affinity for specific cardiac targets.
Heart Targeting Module Development Service
Accelerate Your Targeted Drug Delivery Research!
Are you currently facing challenges in achieving precise drug delivery to the heart, leading to off-target effects and suboptimal therapeutic outcomes? Creative Biolabs' Heart Targeting Module Development helps you overcome these hurdles and accelerate your cardiovascular drug development through advanced module engineering and targeted delivery system design.
Contact our team to get an inquiry now!
Overview
Cardiac dysfunction following ischemic injury remains the primary global mortality driver. Postnatal mammalian myocardium exhibits diminished cardiomyocyte mitotic capacity, rendering ischemic insults (e.g., acute MI) irreparable, culminating in fibrotic remodeling and progressive functional decline. Emerging regenerative strategies—including stem cell-derived interventions, gene-editing platforms, and immunomodulatory/vasculogenic pharmacologics—show preclinical promise in restoring myocardial integrity. However, intravenous delivery of these agents frequently induces off-tissue pharmacodynamics due to circulatory dispersion, driving intense investigation into precision cardiac targeting strategies. Current approaches leverage anatomical access points (coronary/pericardial/epicardial routes) or bioengineered implantables to localize therapeutic bioavailability.
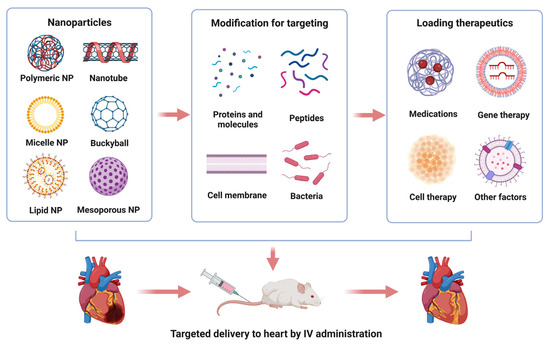 Fig.1 Nanoparticle-based drug delivery for the treatment of myocardial injuries.1,3
Fig.1 Nanoparticle-based drug delivery for the treatment of myocardial injuries.1,3
Delivery System Targeting Heart
Targeted therapeutic delivery remains critical due to its unique capacity to enhance pharmaceutical precision while mitigating collateral biodistribution. Nanoscale platforms dominate this field as primary transport vehicles for bioactive agents. Cardiac vectorization success hinges on three pillars: precise molecular addressing, optimal transport matrices, and engineered binding motifs. Ideal cardiac targeting achieves confined pharmaceutical deposition within myocardial tissues with negligible extra-cardiac exposure post-administration. Current cardiac-targeted systems encompass dendrimeric structures, liposomal assemblies, polymer-drug conjugates, microparticulate systems, nanoparticle subtypes (silica, magnetic, Pt-modified TiO2, polymeric variants), polymeric micelles, and microbubble constructs.
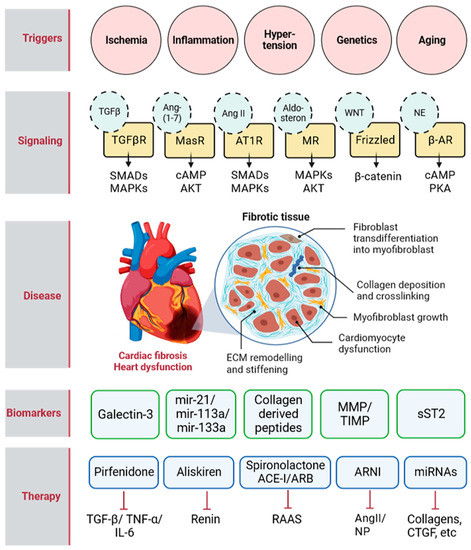 Fig 2. Cardiac fibrosis triggers, signaling pathways, biomarkers, and therapies.2,3
Fig 2. Cardiac fibrosis triggers, signaling pathways, biomarkers, and therapies.2,3
Nanoparticle-based delivery approaches broadly fall into two main types: passive and active targeting. The former leverages the unique physicochemical characteristics of cells and their localized microenvironments. For instance, inflammatory responses and heightened vascular permeability, triggered by cytokines from injured tissues, lead to the enhanced permeability and retention (EPR) phenomenon. In the context of post-myocardial infarction hearts, recent research indicates that nanoparticles can be transported to cardiac tissue through this EPR mechanism. Nevertheless, as vascular permeability normalizes within a few days, the EPR effect proves unsuitable for sustained passive nanoparticle delivery to compromised heart muscle. Another route for passive nanoparticle targeting involves leveraging their capacity to covalently link with circulating cells following surface alterations. These engineered adaptations enable nanocarriers to bind specific blood components, promoting cardiac infiltration. In contrast, active strategies utilize molecular recognition elements targeting cellular markers, ensuring selective binding to cardiomyocyte membrane epitopes or cardiac cell transporters. Examples include nanovectors targeting surface proteins, monoclonal antibodies, peptide motifs, or cytokines serving as MI-specific biomarkers.
What We can Offer?
Creative Biolabs provides a comprehensive suite of products and services to support your Heart Targeting Module Development needs, designed to attract customers seeking advanced solutions in cardiovascular therapeutics.
Custom Heart-Targeting Ligand Design & Synthesis
Advanced Nanoparticle & Liposome Engineering
Design and synthesis of various nanoparticle and liposome formulations (e.g., polymeric nanoparticles, magnetic nanoparticles, stealth liposomes) optimized for cardiac delivery.
Module-Payload Conjugation Services
Expert conjugation of your therapeutic agents to our proprietary or custom-designed targeting modules and carriers.
In vitro & In vivo Efficacy Evaluation
Comprehensive testing of targeting module efficacy, specificity, and safety using advanced in vitro cell models and relevant in vivo animal models of cardiac disease.
Biodistribution & Pharmacokinetic Studies
Detailed analysis of the distribution and clearance of your targeted therapeutics within biological systems.
Experience the Creative Biolabs Advantage - Get a Quote Today
Why Choose Us?
Choosing Creative Biolabs for your Heart Targeting Module Development means partnering with a leader in precision biotechnology. Our commitment to scientific excellence and client success sets us apart.
- Unparalleled Expertise: Years of specialized experience in organ-targeted delivery and cardiovascular biology.
- Cutting-Edge Technology: Access to a diverse array of advanced delivery systems, including dendrimers, liposomes, various nanoparticles (silica, magnetic, polymeric), micelles, microbubbles, and advanced viral/non-viral vectors.
- Precision Targeting Capabilities: Utilization of sophisticated techniques like phage display for isolating highly specific cardiotropic peptides and integration of theranostic approaches for enhanced diagnostics and treatment.
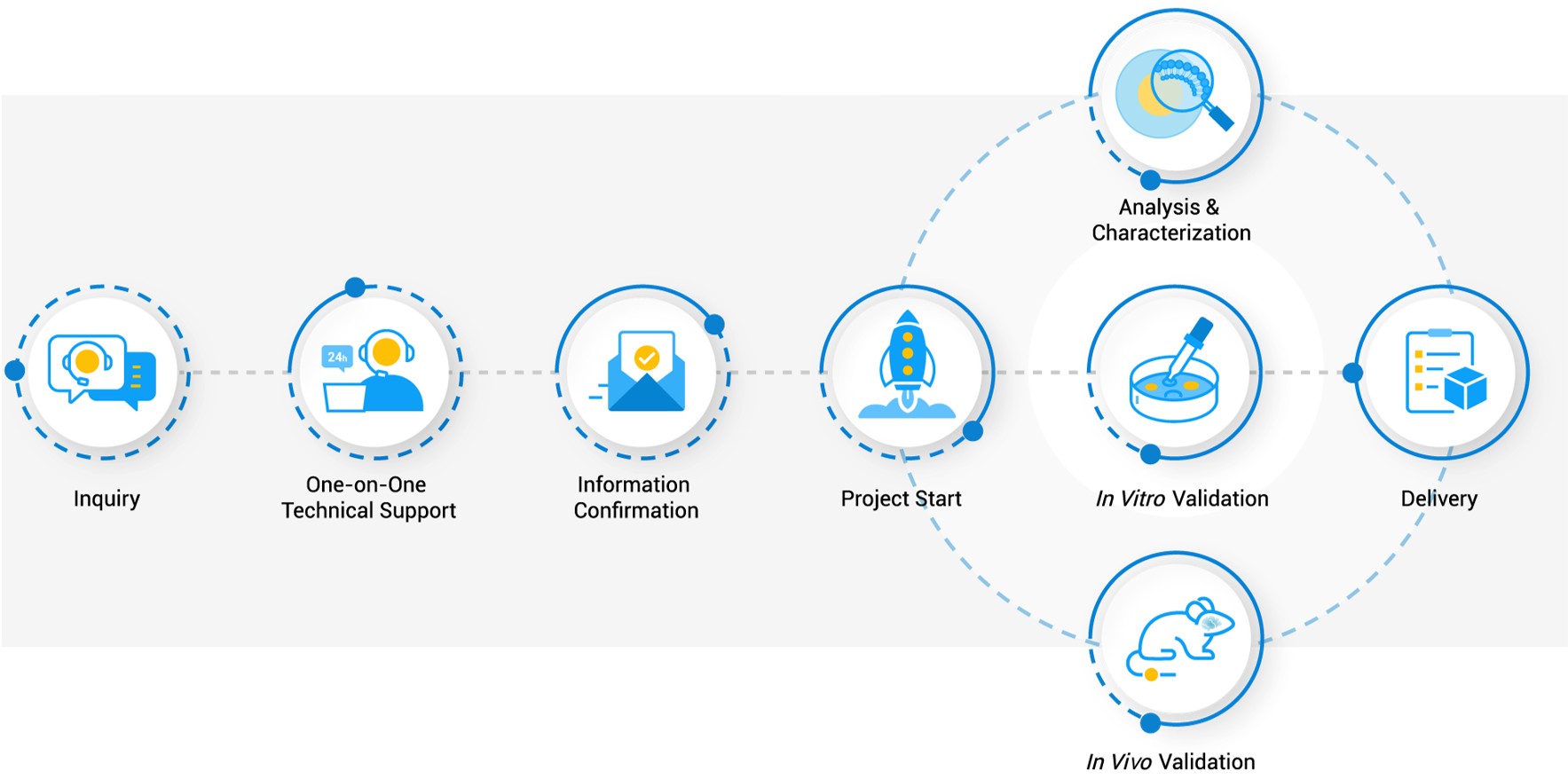
- Comprehensive Workflow: A streamlined, multi-stage process from target identification to in vivo validation, ensuring robust and effective module development.
- Customized Solutions: Ability to develop individual targeting modules or complex module-payload/carrier conjugates tailored to your specific therapeutic needs.
- Demonstrated Success: Our clients consistently achieve significant improvements in cardiac-specific drug accumulation and a notable reduction in systemic toxicity compared to non-targeted approaches.
Workflow
FAQs
Here are some common questions we receive about Heart Targeting Module Development:
How does Creative Biolabs ensure precision in myocardial vectorization?
Our strategy integrates computational ligand engineering targeting cardiovascular biomarkers, followed by multi-tiered cellular binding assays using primary cardiac cultures and non-myocyte counter-screening. Whole-animal biodistribution analytics with automated quantification of myocardial payload deposition and extracardiac signal suppression validate organotropism. Adaptive machine learning-guided refinement cycles continually enhance molecular discrimination parameters.
What types of therapeutic payloads can be delivered using your heart-targeting modules?
Our delivery platforms demonstrate adaptable functionality for vectorizing diverse pharmacological cargos—from low-MW pharmaceuticals to nucleic acid payloads (siRNA, mRNA), gene-editing vectors, peptide/protein biologics, and molecular contrast media. Transport matrices undergo rigorous optimization aligned with cargo biophysical characteristics and required liberation profiles.
What are the primary advantages of using heart-targeted delivery over systemic administration for cardiovascular diseases?
Key benefits involve localized pharmaceutical accumulation in pathological myocardium, driving enhanced treatment response. Simultaneously restricting whole-body biodistribution mitigates non-specific pharmacodynamic interactions while optimizing therapeutic indices. Such targeted vectorization may improve clinical prognosis and enable reduced therapeutic thresholds.
Creative Biolabs provide tailored targeted delivery solutions addressing unique research and therapeutic requirements. To explore these capabilities, please contact us for more information.
References
- Li, Dong, et al. "Nanoparticle Based Cardiac Specific Drug Delivery." Biology 12.1 (2023): 82. doi:10.3390/biology12010082
- Bertaud, Alexandrine, et al. "Signaling pathways and potential therapeutic strategies in cardiac fibrosis." International Journal of Molecular Sciences 24.2 (2023): 1756. doi:10.3390/ijms24021756
- Distributed under Open Access license CC BY 4.0, without modification.



