A comprehensive catalog of pre-formulated Module Delivery Systems (e.g., liposomes, exosomes, polymeric nanoparticles) optimized for CNS delivery, and a selection of validated Targeted Modules (e.g., aptamers, peptides, functionalized lipids, targeted polymers, responsive materials) ready for your astrocyte research and development needs.
Astrocyte Targeting Module Development Service
Are you currently facing challenges in achieving precise drug delivery to the central nervous system, overcoming the blood-brain barrier, or targeting specific glial cell types in neurological research and therapy? Our Astrocytes Targeting Module Development service at Creative Biolabs helps you advance neurological drug discovery, achieve highly specific cellular modulation, and streamline therapeutic development by leveraging advanced ligand design and innovative delivery system engineering.
Overview
Astrocytes, the most abundant glial cells in the central nervous system (CNS), are star-shaped cells that play pivotal and multifaceted roles in brain homeostasis, function, and disease. Far from being mere support cells, astrocytes are highly dynamic and interactive components of the neurovascular unit and tripartite synapse, crucial for neuronal survival, synaptic plasticity, and information processing.
Their basic functions include:
- Neurotransmitter Uptake and Recycling: Astrocytes regulate synaptic neurotransmitter levels, particularly glutamate, preventing excitotoxicity and maintaining proper neuronal signaling.
- Ion Homeostasis: They maintain the ionic balance in the extracellular space, crucial for neuronal excitability.
- Blood-Brain Barrier (BBB) Maintenance: Astrocytes contribute to the integrity and function of the BBB, regulating the passage of substances into and out of the brain.
- Metabolic Support: They provide metabolic substrates (e.g., lactate) to neurons, especially during periods of high activity.
- Synaptic Modulation: Astrocytes release gliotransmitters that can modulate synaptic transmission and plasticity.
- Waste Clearance: They participate in the clearance of metabolic waste products from the brain.
Astrocytes exhibit remarkable plasticity and undergo significant morphological and functional changes, known as "reactive astrogliosis," in response to various CNS insults, including injury, infection, ischemia, and neurodegenerative diseases. While reactive astrocytes can be beneficial in containing damage and scar formation, chronic or aberrant astrogliosis can contribute to pathology by releasing pro-inflammatory mediators, impeding axonal regeneration, or altering neuronal function.
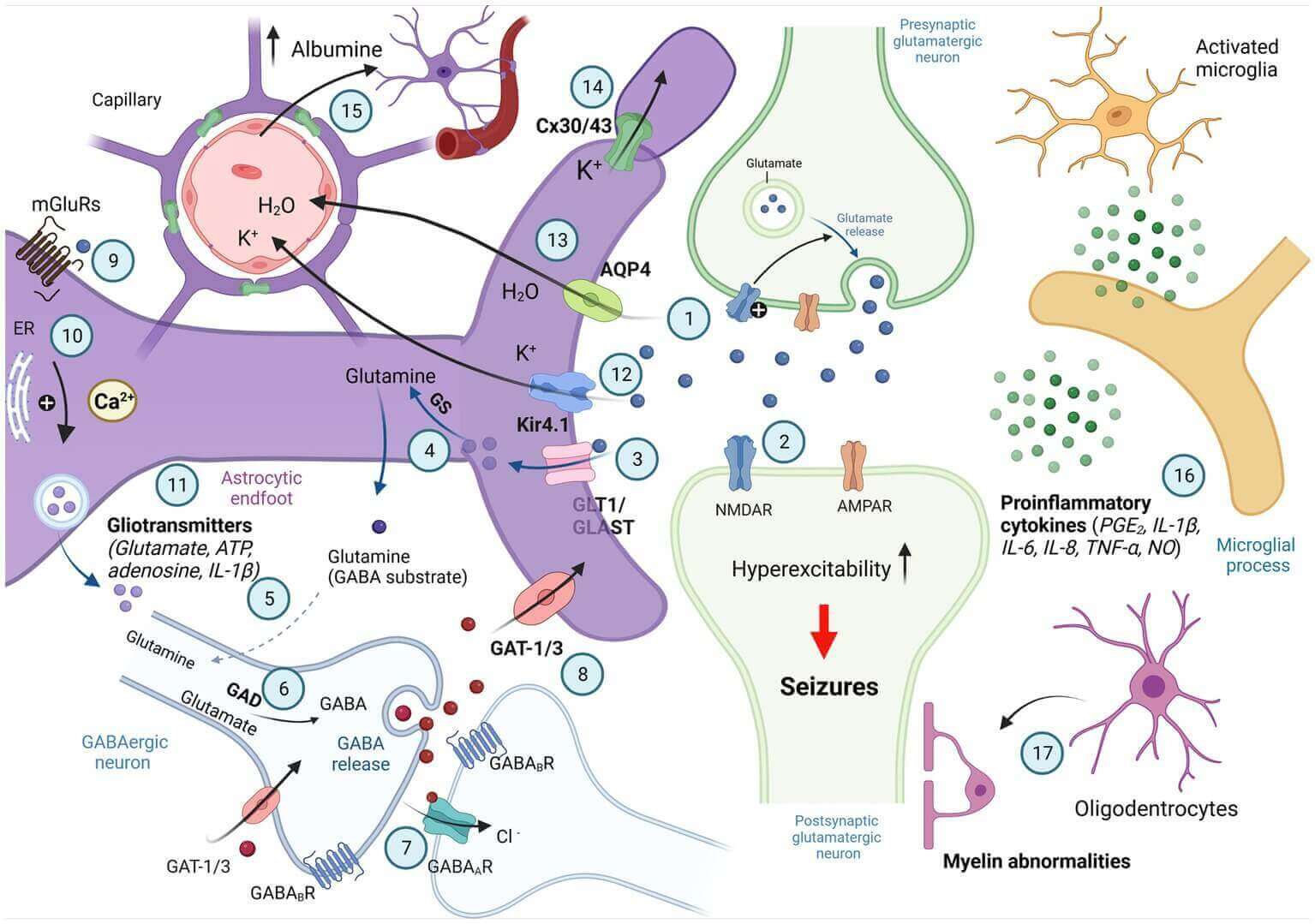 Fig.1 Involvement of astrocytes in synaptic excitability.1.3
Fig.1 Involvement of astrocytes in synaptic excitability.1.3
Key signaling pathways involving astrocytes include those related to calcium signaling, glutamate transport (e.g., GLT-1), water transport (aquaporin-4), and inflammatory pathways (e.g., NF-kappaB, JAK-STAT). Dysregulation of astrocyte function is implicated in a wide array of neurological disorders, including:
- Neurodegenerative Diseases: Alzheimer's disease, Parkinson's disease, Huntington's disease, Amyotrophic Lateral Sclerosis (ALS), where reactive astrocytes contribute to neuroinflammation and neuronal loss.
- Ischemic Stroke: Astrocytes play roles in both neuroprotection and scar formation post-stroke.
- Traumatic Brain Injury (TBI) and Spinal Cord Injury (SCI): Reactive astrocytes contribute to glial scar formation, which can impede axonal regeneration.
- Epilepsy: Astrocytes are involved in maintaining excitatory/inhibitory balance and can contribute to epileptogenesis.
- Brain Tumors: Astrocytes can contribute to the tumor microenvironment, supporting tumor growth and invasion.
Given their central roles in both health and disease, astrocytes represent highly attractive and essential targets for cutting-edge diagnostic and therapeutic strategies aimed at modulating CNS pathologies.
Creative Biolabs' Astrocytes Targeting Solution
Creative Biolabs' precision delivery platform for neurological applications is built around its astrocyte targeting modules. These engineered components integrate into delivery systems like lipid nanoparticles, liposomes, or exosomes. The systems are then functionalized with specific ligands that bind to receptors on astrocytes.
Uptake by astrocytes typically involves both:
Passive targeting
Nanoparticles (100-200 nm for compromised vasculature, smaller for BBB penetration) can accumulate in areas like brain tumors or neuroinflammation, or passively cross the blood-brain barrier, then be internalized by astrocytes.
Active targeting
Ligands on the delivery system bind to specific astrocyte surface markers, triggering receptor-mediated endocytosis for highly selective payload delivery.
This modular design offers flexibility for diverse neuroscience research and therapeutic goals.
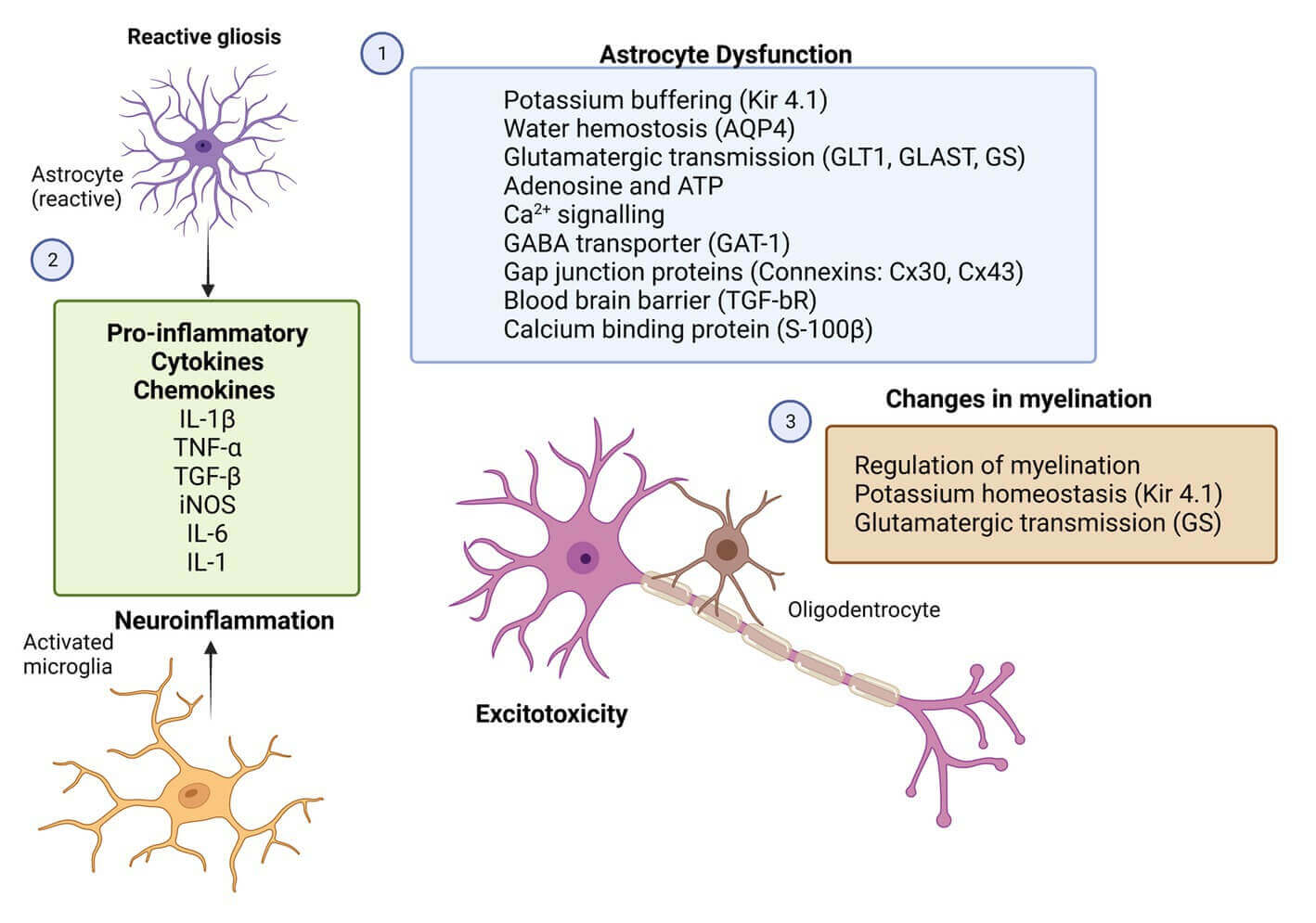 Fig.2 Potential therapeutic targets for glial cells in epilepsy.1,3
Fig.2 Potential therapeutic targets for glial cells in epilepsy.1,3
Astrocytes Targeting Module
Creative Biolabs' sophisticated microglia targeting modules precisely guide delivery systems to specific microglial types or activation states via high-affinity binding to unique surface markers. This ensures therapeutic payloads accumulate accurately in the CNS, maximizing efficacy and minimizing off-target effects. Our diverse module library offers flexibility, with each type selected for specificity, stability, and easy conjugation. Modules typically combine a high-affinity ligand (e.g., peptide, antibody) with a stable linker to a delivery vehicle (e.g., nanoparticles), allowing customization for neurological projects.
| Ligand Type | Mechanism of Action | Targeted Marker(s) | Advantages/Application |
|---|---|---|---|
| Antibodies | Highly specific proteins binding unique microglial antigens. | FAP, S100B, GLT-1, AQP4, CD44, Vimentin, Aldh1L1, EAAT1/2, GPR56, LRP1, P2Y1R, P2Y2R, P2X7R. | Unparalleled precision and validated development trajectories. |
| Peptide | Short amino acid sequences binding specific microglial receptors. | RGD peptide (targeting integrins), ApoE-derived peptides (for LRP1), Angiopep-2 (for LRP1), Transferrin receptor-binding peptides, specific peptides for astrocyte-specific receptors. | Compact dimensions, effective tissue permeation, extensive customization capacity, and favorable cost-efficiency. |
| Carbohydrates | Glycan structures interacting with microglial lectins. | Mannose receptors (e.g., CD206, if expressed on specific astrocyte subsets), other C-type lectin receptors. | Utilize inherent cellular internalization mechanisms. Applicable for engaging astrocytes exhibiting particular metabolic activities or inflammatory phenotypes. |
| Aptamers | DNA/RNA molecules binding specific microglial targets with high affinity. | Various astrocyte-specific surface proteins or receptors. | Superior binding selectivity, minimal immune response induction, straightforward chemical synthesis and adaptation. |
| Other | Diverse mechanisms (receptor binding, ion channel modulation). | Folate receptor (FR), growth factor receptors (e.g., EGFR, FGFR), specific transporters or ion channels. | Stable incorporation within carrier platforms, enabling exact regulation of ligand density and spatial orientation. |
Contact Us About Astrocytes Targeting Module
What We Can Offer?
Creative Biolabs is uniquely positioned at the forefront of targeted drug delivery innovation, extending our expertise to the intricate landscape of the central nervous system. Our team of expert biologists, chemists, and engineers brings over two decades of collective experience in developing sophisticated delivery solutions, now specifically tailored for astrocyte targeting.
Ready-to-Use Products
Customized Services
Our bespoke service allows us to develop tailored delivery systems and novel targeted modules from concept to validation, precisely meeting your project's unique specifications for astrocyte targeting. This includes custom aptamer, peptide, or polymer synthesis and conjugation, as well as optimization of delivery system characteristics for specific astrocyte subsets or disease contexts.
Conjugation Services
Expertise in conjugating selected ligands to various delivery platforms (nanoparticles, liposomes, polymers, etc.) for efficient astrocyte targeting.
Pre-Clinical Validation
Robust in vitro and in vivo testing to assess targeting efficiency, cellular uptake, biodistribution within the CNS, and therapeutic efficacy in relevant neurological models.
Comprehensive Scientific Support
Partner with us to leverage our deep scientific knowledge, state-of-the-art facilities, and rigorous quality control for your astrocyte targeting projects, from experimental design to data analysis.
Workflow

Why Choose Us?
Partnering with Creative Biolabs means choosing a path to accelerated neurological drug development, enhanced therapeutic efficacy, and a significant reduction in off-target effects within the brain. Our commitment to innovation and scientific excellence ensures that your therapeutic agents reach their astrocyte targets with unprecedented precision, unlocking new possibilities for treating neurological diseases.
Proven Expertise
Our team of highly specialized biologists, chemists, and engineers possesses deep scientific knowledge in drug delivery systems and targeting module development, with specific insights into CNS biology and astrocyte function.
Innovative Technology
We leverage state-of-the-art platforms for module synthesis, conjugation, and characterization, ensuring the highest quality and performance for astrocyte-specific targeting.
Bespoke Adaptation & Versatility
We provide personalized aptamer/peptide engineering and carrier platform refinement to fulfill your distinct treatment objectives and microglial targeting specifications, including for distinct microglial phenotypic conditions.
Exacting Standards & Consistency
Our adherence to methodological rigor assures dependable, repeatable, and premium outcomes for pivotal initiatives, spanning initial conception through preclinical substantiation.
Published Data
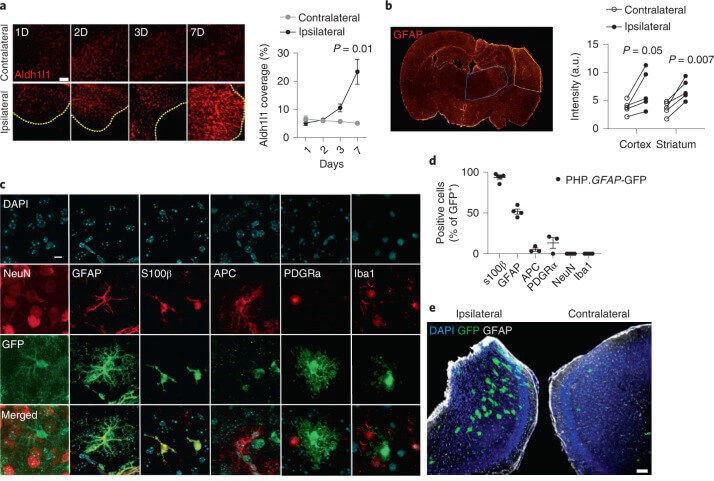 Fig.3 Synthetic delivery to the brain via a dual-lock gene-delivery system.1,3
Fig.3 Synthetic delivery to the brain via a dual-lock gene-delivery system.1,3
The researchers developed a gene-delivery approach using AAV-PHP.B.GFAP to specifically deliver IL-2 to astrocytes in the brain. This method successfully increased brain IL-2 production three-fold, leading to a corresponding rise in brain Treg cell frequency and absolute number in a dose-dependent manner, restricted to the brain without peripheral expansion. The treatment was well-tolerated, showing no major off-target effects, impact on neuronal or astrocyte function, or behavioral abnormalities, and maintained blood-brain barrier integrity. Experimentally, the PHP.GFAP-IL-2 treatment reduced lesion size in a controlled cortical impact model of traumatic brain injury (TBI) and in a secondary stroke model when administered after the primary stroke. It also significantly reduced the cumulative clinical score and showed a protective effect in a curative experimental autoimmune encephalomyelitis (EAE) model, even after clinical onset. This suggests a promising "dual-lock" approach for treating neuroinflammatory pathologies by increasing local brain IL-2.
FAQs
Q: What types of payloads can your astrocyte targeting modules deliver?
A: Our versatile targeting modules are designed to precisely deliver a wide range of therapeutic and diagnostic agents to astrocytes. This includes small molecule drugs, various nucleic acids (such as siRNA, mRNA, and gene-editing components), proteins, and imaging contrast agents. Our approach is highly adaptable to accommodate diverse payload types for your specific neurological research or therapeutic applications.
Q: How do you ensure the specificity of your targeting modules for astrocytes versus other brain cells?
A: We employ a multi-faceted approach to ensure high specificity. This involves rigorous selection of ligands that bind to markers uniquely or highly expressed on astrocytes, coupled with advanced engineering of the delivery system to optimize its interaction with the target cells. Our validation processes include comprehensive in vitro and in vivo studies to confirm selective uptake by astrocytes and minimal off-target effects on neurons or other glial cells.
Q: Can your targeting modules differentiate between healthy and reactive astrocytes?
A: Yes, absolutely. Astrocytes undergo significant phenotypic changes during disease, expressing different surface markers. Our expertise extends to developing highly specific targeting modules that can differentiate between various astrocyte states, including quiescent and reactive populations. By leveraging these distinct expression profiles, we can design solutions for highly nuanced and precise therapeutic interventions targeting specific pathological astrocytes.
Q: Are your astrocyte targeting modules compatible with different drug delivery systems?
A: Absolutely. Our module design considers compatibility with various drug delivery platforms from the outset. Whether you prefer liposomes, lipid nanoparticles (LNPs), polymeric nanoparticles, or exosomes, we can provide effective coupling strategies and technical support to equip your chosen delivery vehicle with astrocyte-specific targeting capabilities, aiming for enhanced drug delivery efficiency and therapeutic outcomes in the CNS.
Creative Biolabs stands as your premier partner in advancing neurological research and therapy through our cutting-edge Astrocytes Targeting Module Development Services. Our commitment to innovation, scientific rigor, and tailored solutions ensures that your therapeutic agents reach their astrocyte targets with unparalleled precision, unlocking new possibilities for disease treatment and fundamental neuroscience discoveries. We offer a comprehensive suite of services, from ready-to-use products to bespoke module design and rigorous pre-clinical validation, all supported by our team's deep expertise.
Contact our expert team today to discuss your specific project needs and discover how Creative Biolabs' Astrocytes Targeting Module Development can accelerate your path to success.
References
- Yshii, Lidia et al. "Astrocyte-targeted gene delivery of interleukin 2 specifically increases brain-resident regulatory T cell numbers and protects against pathological neuroinflammation." Nature immunology vol. 23,6 (2022): 878-891. DOI:10.1038/s41590-022-01208-z.
- Çarçak, Nihan et al. "Astrocytes as a target for therapeutic strategies in epilepsy: current insights." Frontiers in molecular neuroscience vol. 16 1183775. 31 Jul. 2023, DOI:10.3389/fnmol.2023.1183775.
- Distributed under Open Access license CC BY 4.0, without modification.



