Lysosome Targeting Module Development Service
Accelerate Your Targeted Drug Delivery Research!
Are you currently facing challenges in delivering your therapeutic payloads to specific intracellular locations? Do you struggle with off-target effects and suboptimal drug efficacy? Creative Biolabs' Lysosome Targeting Module Development service helps you achieve precise and efficient delivery of your molecules to lysosomes, enhancing therapeutic efficacy and minimizing adverse effects through advanced targeting technologies.
Contact our team to get an inquiry now!Overview
As key degradative hubs, lysosomal compartments maintain macromolecular recycling through their proton-rich lumen housing six dozen hydrolytic enzymes. Substrate processing occurs through autophagic pathways for endogenous material or vesicular trafficking for extracellular cargo via phagocytic/endocytic routes. Over 50 inherited monogenic disorders—primarily categorized as lysosomal storage pathologies—stem from functional impairments in these organelles. Substrate accumulation from compromised degradation cascades triggers cellular dysfunction, exemplified by Niemann-Pick disease and Hurler syndrome. Beyond classical storage disorders, lysosomal anomalies correlate with neurodegenerative conditions like Alzheimer's and paradoxical roles in autoimmune disease modulation.
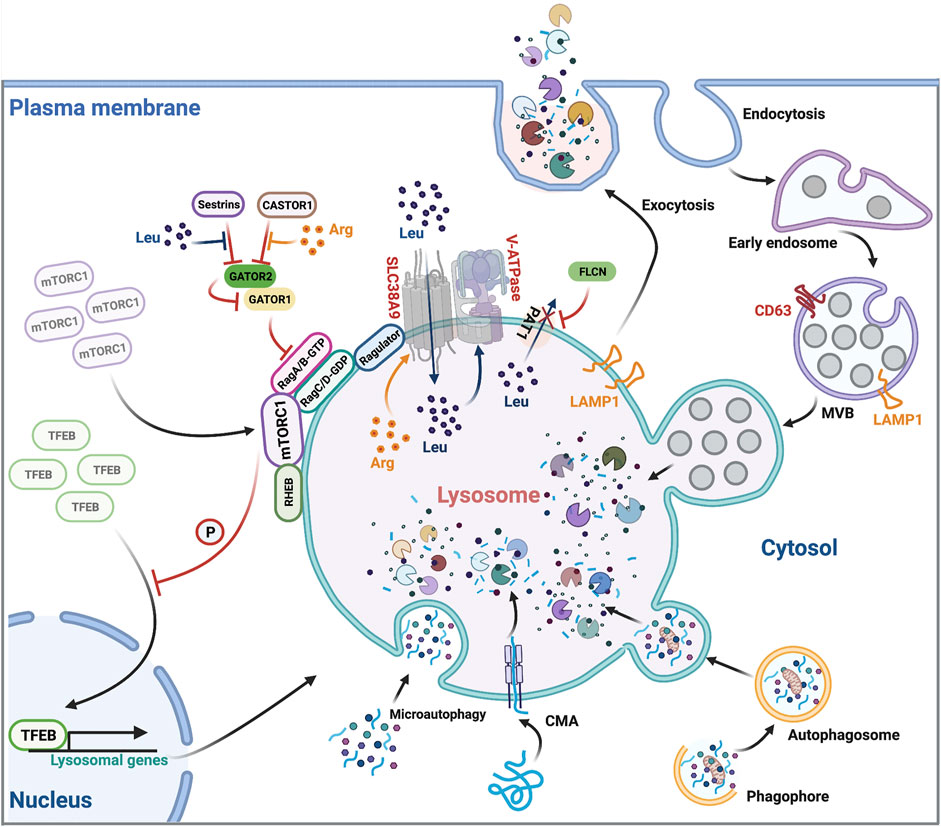 Fig.1 Overview of lysosomal function.1,3
Fig.1 Overview of lysosomal function.1,3
Lysosome Targeting Strategy
Lysosomotropic delivery typically employs extracellular receptor modules—including transferrin receptors, folate transporters, VEGF interactors, and LDL recognition systems—for cellular uptake guidance. The lysosomal proteolytic apparatus integrates both soluble and membrane-bound components initially synthesized through cytosolic pathways. Structural analyses confirm mannose-6-phosphate serves as the principal structural determinant for lysosomal protein trafficking.
- Target lysosomal acidification
The lysosomal proton gradient serves as both a defining organellar feature and operational foundation for functional regulation. Hypoacidic conditions prove critical for sustaining oncogenic metabolic demands and autoimmune cell hyperactivation, whereas neurodegenerative and cardiovascular pathologies demonstrate compromised proton homeostasis and autophagic flux. Therapeutic targeting modalities can thus be calibrated to specific pH-dependent lysosomal profiles.
- Target lysosomal cathepsins
As principal proteolytic effectors, lysosomal cathepsins critically mediate degradative processes within these organelles. Substantiated research confirms their mechanistic role in advancing oncogenesis—including tumor cell proliferation, metastatic invasion, neovascularization, and chemoresistance—with upregulated enzymatic activity and expression profiles observed across leukemia subtypes and solid malignancies. These enzymes represent validated molecular targets for therapeutic intervention in cancer, neurodegenerative pathologies, and autoimmune dysregulation.
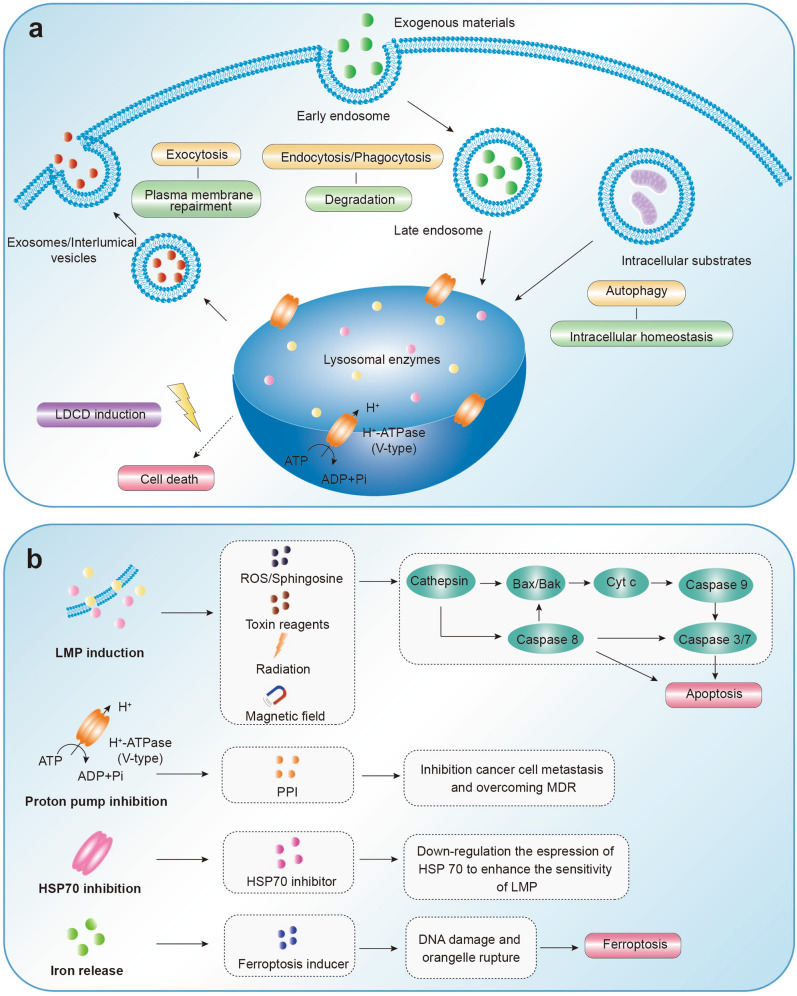 Fig 2. The personalized therapeutic strategy toward lysosomes.2,3
Fig 2. The personalized therapeutic strategy toward lysosomes.2,3
- Target lysosomal membrane permeability and integrity
Physiological stressors trigger lysosomal membrane permeabilization (LMP) or complete organellar fragmentation, dispersing hydrolytic enzymes into the cytoplasm that initiate pro-inflammatory cascades and regulated cell death. This destabilization thus exhibits dual pathophysiological relevance—propagating inflammatory pathogenesis while concurrently presenting druggable vulnerabilities for oncological targeting.
- Target lysosomal calcium signaling
Functioning as central calcium regulatory hubs, lysosomes utilize Ca²⁺ to coordinate organelle genesis, proton gradient maintenance, structural reorganization, and vesicular processes like autophagy and endocytic transport. Mounting data reveal their pivotal role in tumorigenesis and neural degradation cascades. TRPML and TPC ion channels—exclusively localized to endolysosomal membranes—are now classified as prime therapeutic candidates for pharmacological modulation in mammalian systems.
- Target mTOR signaling
Lysosomes function as catalytic hubs for mTOR signaling element assembly, spatial organization, and activation cascades, while this kinase reciprocally modulates lysosomal catabolic processes through nutrient-sensing mechanisms. These organelles and mTOR kinase operate through a bidirectional regulatory axis governing metabolic coordination. Aberrant overactivation of mTOR-driven signaling cascades—key regulators of cell growth and metabolic reprogramming—is frequently observed in malignancies, establishing mTOR inhibitors as precision therapeutic agents in oncological interventions.
What We can Offer?
Creative Biolabs has a complete module delivery system and an experienced team of scientists. We offer:
- Individual targeting modules.
- Different types of module-payload/carrier complexes for specific subcellular organelles.
- A wide range of corresponding products.
- In vitro and in vivo validation of targeting module efficacy and specificity.
- Consultation and support throughout the project lifecycle.
Experience the Creative Biolabs Advantage - Get a Quote Today
Why Choose Us?
Creative Biolabs is a leading provider of lysosome-targeting solutions, offering unparalleled expertise and cutting-edge technologies to accelerate your research and development efforts. We are committed to delivering high-quality products and services with exceptional customer support.
- Expertise: Creative Biolabs has a team of experienced scientists with extensive knowledge in lysosomal biology, drug delivery, and bioconjugation chemistry.
- Customization: We offer tailored solutions to meet your specific research needs, from designing novel targeting modules to developing customized conjugates.
- Advanced Technology: Creative Biolabs utilizes state-of-the-art technologies and platforms to ensure the highest quality and efficiency in our services.
- Published Data: Our work is supported by strong scientific evidence and Published Data, demonstrating the efficacy and reliability of our lysosome-targeting solutions.
-
Core Service Advantages:
- Enhanced hemocompatibility (systemic circulation stability)
- Minimized off-target cellular uptake
- Optimized membrane permeability profiles
- Facilitated endosomal egress mechanisms
- Customizable modular combinatorial design
Workflow

FAQs
How specific are Creative Biolabs' lysosome-targeting modules?
Our modules are designed for high specificity to ensure minimal off-target effects. We employ rigorous validation procedures to confirm their selectivity.
Can Creative Biolabs customize targeting modules for my specific payload?
Yes, we offer full customization services. Our team will work closely with you to design and synthesize a targeting module tailored to your unique payload and research goals.
What are the advantages of using lysosome-targeted delivery compared to traditional drug delivery methods?
Lysosome-targeted delivery offers enhanced drug efficacy, reduced off-target toxicity, and the ability to modulate specific lysosomal functions. This approach can be particularly beneficial for treating diseases with lysosomal involvement.
What factors should I consider when choosing a lysosome-targeting module?
Key factors include the target receptor, payload properties, desired cellular uptake, and lysosomal release kinetics. Our experts can guide you through the selection process to ensure optimal results.
What kind of data do you provide to support the efficacy of your lysosome-targeting modules?
We provide comprehensive data packages, including in vitro and in vivo studies, demonstrating the specificity, efficacy, and safety of our targeting modules. Specific data will vary depending on the project.
Creative Biolabs maintains the life science sector's most comprehensive service array for lysosome targeting solutions. Please contact us for more information.
References
- Jin, Jun, et al. "Lysosomes in T Cell Immunity and Aging." Frontiers in aging 2 (2021): 809539.
- Yang, Jingjing, et al. "Organelle-targeted therapies: a comprehensive review on system design for enabling precision oncology." Signal transduction and targeted therapy 7.1 (2022): 379.
- Distributed under Open Access license CC BY 4.0, without modification.



