Development and provision of highly specific targeting ligands, including peptides, antibodies, aptamers, or small molecules, designed to bind selectively to diseased joint tissues or cells.
Joint Targeting Module Development Service
Accelerate Your Targeted Drug Delivery Research!
Are you currently facing challenges such as rapid drug clearance, systemic toxicity, or limited bioavailability, hindering the achievement of long-term therapeutic response in joint diseases? Our Joint Targeting Module Development service helps you enable precise, long-term therapeutic delivery and overcome traditional drug limitations through advanced targeted delivery systems and innovative nanomedicine approaches. Creative Biolabs' proprietary module development ensures the restoration of joint structure and function with unparalleled precision.
Contact our team to get an inquiry now!
Overview
Rheumatoid arthritis (RA) is a persistent systemic autoimmune disorder characterized by progressive inflammatory joint deterioration and multi-tissue damage. The condition originates from dysregulated immune responses, driving localized lesions in articular cartilage, osseous tissues, tendons, and ligaments. Contemporary research has achieved significant breakthroughs in decoding its pathophysiological drivers. Central to RA pathomechanisms is pro-inflammatory transformation of synovial membranes lining movable joints, tendon sheaths, and bursal cavities. Cytokine-primed macrophages initiate synovial hyperplasia, chondrocyte apoptosis, bony erosions, mobility limitations, and articular rigidity. Progressive articular cartilage breakdown and subchondral bone loss ensue through matrix metalloproteinase cascades and osteoclastic resorption, culminating in irreversible joint deterioration. Despite high prevalence, persistent knowledge gaps in RA pathogenesis continue to pose significant treatment challenges.
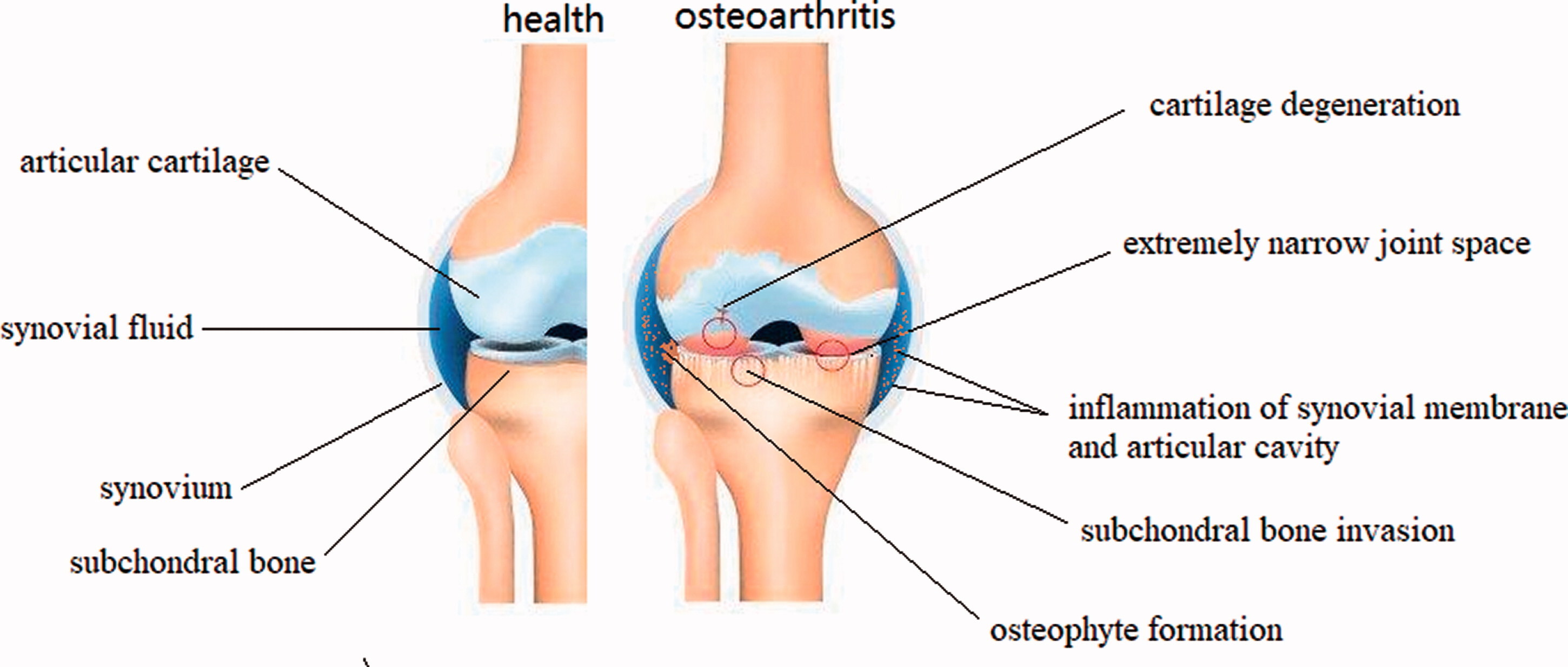 Fig.1 Schematic representation of healthy knee joint structure and pathological changes of knee osteoarthritis.1,3
Fig.1 Schematic representation of healthy knee joint structure and pathological changes of knee osteoarthritis.1,3
Delivery System Targeting Joint
Standard pharmacological agents for RA management encompass NSAIDs, corticosteroids, and DMARDs. However, non-selective biodistribution and poor tissue targeting via parenteral delivery frequently induce off-target toxicity. Elevated dosage regimens necessitated by rapid clearance rates and subtherapeutic drug accumulation at pathological sites exacerbate both treatment-related complications and economic burdens. Emerging nanoscale drug delivery paradigms, including engineered nanoparticles, address these limitations through enhanced pharmacokinetic control.
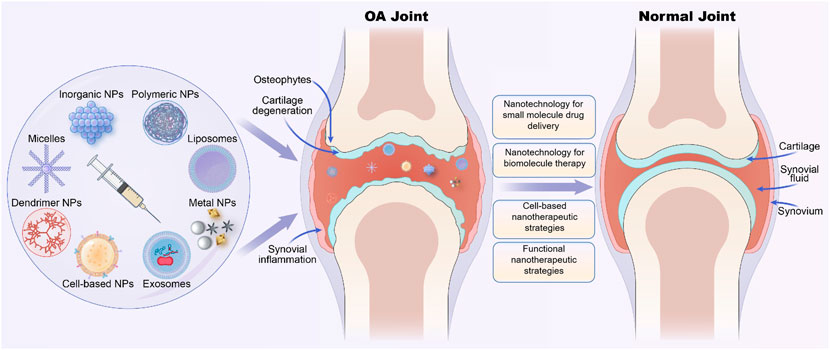 Fig.2 Various nano-therapeutic strategies based on different nanoparticles for osteoarthritis (OA) treatment.2,3
Fig.2 Various nano-therapeutic strategies based on different nanoparticles for osteoarthritis (OA) treatment.2,3
Example of Targeted Delivery System
Folate receptor β (FR-β) exhibits selective overexpression on activated macrophages, with minimal expression in healthy tissues. In RA therapy, MTX-loaded liposomes conjugated via SP-DS3 peptide linkers to FR-β demonstrated enhanced macrophage-specific uptake in FR-β+ cells. These folate-functionalized vesicles exhibited preferential articular accumulation in CIA models, showing prophylactic efficacy against arthritis induction. FR-β targeting was further applied to albumin nanoparticles, improving ETX delivery specificity in carrageenan-induced rat arteritis. Concurrently, HA-mediated active targeting platforms have emerged for RA treatment. HA-5b-CA polymeric micelles encapsulating DAPT displayed elevated Cy5.5-labeled nanoparticle internalization in activated versus resting macrophages, validating their active targeting capability.
What We can Offer?
Creative Biolabs is equipped with a complete module delivery system and an experienced team of scientists, ready to meet your specific project needs. We provide a wealth of corresponding products and services designed to deliver targeted therapeutic solutions for joint diseases:
Individual Targeting Modules
Module-Payload/Carrier Complexes
Construction of various sophisticated complexes, incorporating your therapeutic payload with optimized targeting modules and diverse carrier systems such as nanoparticles, liposomes, or polymeric microparticles.
Customized Module Development for Specific Subcellular Organelles
Tailored solutions for precise delivery of therapeutic agents to specific subcellular compartments within joint cells, enabling highly targeted interventions.
Comprehensive In Vitro Evaluation
Rigorous in vitro assessment of targeting module efficacy, specificity, binding affinity, and cellular uptake kinetics using advanced cell-based assays and imaging techniques.
Robust In Vivo Preclinical Evaluation
Extensive in vivo studies in relevant animal models to evaluate joint accumulation, residence time, biodistribution, and therapeutic efficacy in mitigating disease progression and symptoms.
Consultation and Project Design Support
Expert guidance from initial concept to project completion, ensuring the most effective and efficient development pathway for your targeted therapy.
Experience the Creative Biolabs Advantage - Get a Quote Today
Why Choose Us?
Creative Biolabs stands at the forefront of Joint Targeting Module Development, offering unparalleled expertise and a commitment to innovation that sets us apart. Our advantages ensure your project's success:
- Decades of Expertise: Over years of specialized experience in targeted delivery systems, ensuring deep scientific knowledge and practical problem-solving.
- Comprehensive Platform: A complete module development system, from initial design to in vivo validation, offering end-to-end solutions.
- Validated Performance: Documented achievements in resolving intricate pharmaceutical delivery obstacles across multiple pharmacological classes.
- Anatomically Targeted Delivery: Engineered for spatial accuracy in therapeutic deposition, significantly reducing off-target exposure.
- Temporal Release Engineering: Specialized in creating delivery platforms with extended pharmaceutical retention and chronotherapeutic effects.
- State-of-the-Art Evaluation: Robust in vitro and in vivo capabilities for thorough characterization and optimization of targeting modules.
- Collaborative Approach: A highly experienced team of scientists dedicated to working closely with you, providing tailored solutions.
Workflow
FAQs
Here are some common questions we receive about Joint Targeting Module Development at Creative Biolabs:
How do Creative Biolabs' joint targeting modules differ from conventional intra-articular injections?
Our platforms are designed with improved targeting precision and extended retention duration. Contrasting conventional intra-articular injections prone to quick medication elimination, our site-specific delivery mechanisms attach precisely to affected cellular structures in joints, sustaining effective drug levels over extended periods and diminishing administration frequency. This results in prolonged clinical efficacy and reduced whole-body distribution.
Which pharmacological compounds are compatible with your delivery modules?
Our multi-agent delivery architecture supports diverse therapeutics: low-MW pharmaceuticals, biopharmaceuticals (monoclonal antibodies, recombinant proteins), and nucleic acid payloads (siRNA/mRNA constructs). We implement precision biofunctionalization protocols to ensure optimal incorporation of specialized cargo into engineered transport mechanisms.
Is your platform adaptable for particular articular pathologies extending beyond RA and OA?
Certainly. Though RA and OA represent primary implementation areas, our Joint-Specific Targeting Platform features structural configurability. We tailor recognition elements and transport mechanisms for distinct articular disorders requiring specialized biomolecular signatures. We invite consultation with our specialists regarding your project’s unique therapeutic objectives.
Creative Biolabs provide tailored targeted delivery solutions addressing unique research and therapeutic requirements. To explore these capabilities, please contact us for more information.
References
- Mao, Liwei, et al. "Targeted treatment for osteoarthritis: drugs and delivery system." Drug Delivery 28.1 (2021): 1861-1876. doi:10.1080/10717544.2021.1971798
- Guo, Xinjing, et al. "Recent advances in nano-therapeutic strategies for osteoarthritis." Frontiers in Pharmacology 13 (2022): 924387. doi:10.3389/fphar.2022.924387
- Distributed under Open Access license CC BY 4.0, without modification.



