We conduct robust safety assessments and toxicology studies in accordance with regulatory guidelines to generate the data necessary for your IND application.
IND-Enabling Study & GMP Manufacturing Support Platform
Are you currently facing the complex challenges of navigating the preclinical and clinical stages of drug development, including lengthy timelines and stringent regulatory requirements? Creative Biolabs' comprehensive IND-Enabling Studies & GMP Manufacturing Support services help you streamline the path from discovery to clinical trials. We provide high-quality manufacturing support and robust preclinical data through our advanced scientific platforms, ensuring your drug candidate is primed for regulatory success.
IND-Enabling Studies and GMP Manufacturing Support
The journey of a drug from the lab to a patient is a long and complex one, governed by strict regulatory oversight. At the heart of this process are IND-Enabling Studies and Good Manufacturing Practice (GMP). The purpose of IND-Enabling studies is to generate the robust preclinical data required to prove a new drug is safe and unlikely to cause harm in humans, a prerequisite for obtaining an Investigational New Drug (IND) application from regulatory bodies. These studies are the final step in preclinical development, providing the essential evidence to bridge the gap to human clinical trials. Concurrently, GMP manufacturing provides the quality assurance framework for the consistent production of the "test articles" used in these studies. This ensures that the results of preclinical toxicology and safety assessments are reliable and that the drug used in clinical trials is identical in quality to the one produced for early studies.
IND-enabling studies and GMP manufacturing are intrinsically linked, representing a critical juncture in the drug development pipeline. The efficacy and safety of a drug can only be accurately assessed if the test material is of a known and consistent quality, which is the primary purpose of GMP. Our integrated approach at Creative Biolabs ensures both.
The Role of IND-Enabling Studies
- Safety and Toxicology Data: These studies are designed to collect crucial data on a drug candidate's safety profile, which is a key requirement for regulatory submission.
- Preclinical Bridge to the Clinic: They serve as the final step in preclinical development, providing the essential evidence needed to justify testing the drug in humans.
- Foundational for Regulatory Success: The results of these studies are the core of the Investigational New Drug (IND) application, which must be approved before human trials can begin.
The Importance of GMP Manufacturing Support
- Ensuring Product Quality: GMP establishes a system of controls over raw materials, facilities, equipment, and personnel to ensure the drug product is consistently high-quality.
- Minimizing Risk: Adherence to GMP minimizes the risks of contamination, mix-ups, and errors during the production process.
- Providing Reliable Test Articles: GMP ensures the test articles used in IND-enabling studies are consistent and reliable, making the study results scientifically valid and defensible.
Application
The combined support of IND-enabling studies and GMP manufacturing is applicable to a wide range of therapeutic development programs, particularly in the fields of targeted drug delivery and biologics. These services are essential for:
- Targeted Delivery Systems: Developing and validating nanoparticle, liposome, and exosome-based delivery systems for small molecules, nucleic acids, and proteins, ensuring they are manufactured consistently for preclinical and clinical use.
- Biologics Development: Manufacturing complex molecules such as recombinant hyperimmune globulins, monoclonal antibodies, and cell therapies under GMP conditions to ensure sterility, consistency, and potency.
- Gene Therapy: Supporting the production of viral vectors and other gene delivery platforms, where consistent quality is paramount for both safety and efficacy.
- Vaccine Development: Ensuring the scalable and high-quality manufacturing of vaccine components for preclinical testing and clinical trials.
Contact Us About Bioconjugation Services
What We Can Offer?
Creative Biolabs offers a comprehensive suite of services designed to support your drug development journey from early-stage research to clinical readiness. Our unique position at the forefront of pharmaceutical innovation allows us to provide:
IND-Enabling Studies
GMP Manufacturing Support
Our facilities provide consistent, high-quality manufacturing of test articles and drug products for both preclinical studies and clinical trials, adhering to strict Good Manufacturing Practice (GMP) standards.
Ready-to-Use Products
A catalog of pre-formulated delivery systems (liposomes, exosomes, LNPs) and a selection of validated Targeted Modules (aptamers, peptides) are available for your research and development needs.
Customized Services
Our bespoke service allows us to develop tailored delivery systems and novel targeted modules from concept to validation, precisely meeting your project's unique specifications.
Conjugation Services
Expertise in conjugating selected ligands to various delivery platforms (nanoparticles, liposomes, polymers, etc.).
Pre-Clinical Validation
We offer in vitro and in vivo testing to assess targeting efficiency, cellular uptake, biodistribution, and therapeutic efficacy.
Workflow

Why Choose Us?
Partnering with Creative Biolabs means choosing a path to accelerated drug development, enhanced therapeutic efficacy, and a significant reduction in off-target effects. Our commitment to innovation and scientific excellence ensures your therapeutic agents are handled with unprecedented precision, unlocking new possibilities for disease treatment.
Proven Expertise
Our team of highly specialized biologists, chemists, and engineers possesses deep scientific knowledge in drug delivery systems and GMP manufacturing.
Innovative Technology
We leverage state-of-the-art platforms for module synthesis, conjugation, and characterization. Published Data from our research demonstrates the success and reliability of our methods.
Tailored Customization & Flexibility
We offer customized aptamer/peptide design and delivery system optimization for your specific therapeutic goals.
Rigorous Quality & Reliability
Our commitment to scientific rigor and adherence to GMP standards ensure reliable, reproducible, and high-quality results for your critical projects.
Published Data
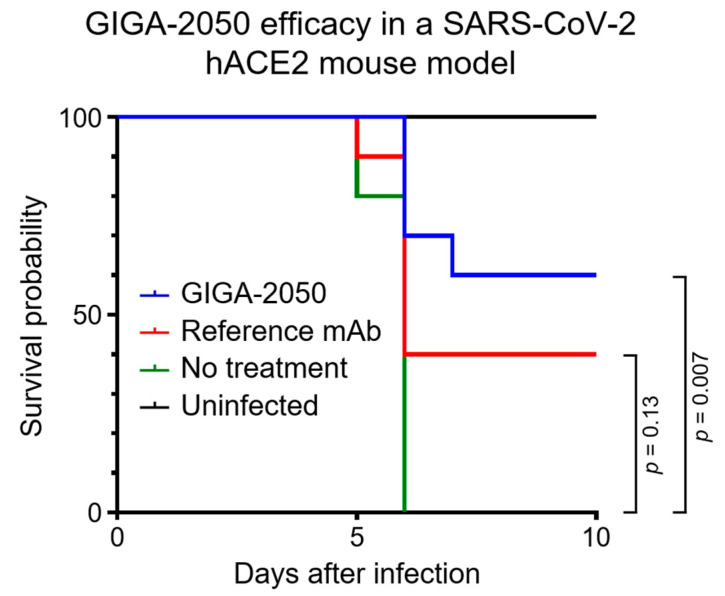 Fig.1 Kaplan–Meier curves for K18 hACE2 transgenic mice after SARS-CoV-2 infection.1
Fig.1 Kaplan–Meier curves for K18 hACE2 transgenic mice after SARS-CoV-2 infection.1
Published data from a study on the GMP manufacturing and IND-enabling studies for GIGA-2050, a recombinant hyperimmune globulin targeting SARS-CoV-2, demonstrates the successful development of this new class of drugs. The study aimed to prove that such drugs could be consistently manufactured at a clinical scale, offering a scalable alternative to traditional plasma-derived products, which have limitations in procurement and potency.
The experiments focused on several key aspects of the manufacturing and preclinical process. The stability of the GIGA-2050 cell line was assessed, showing it was robust for a significant number of generations. The consistency of the downstream manufacturing process was also verified by comparing impurity clearance in two different facilities. A comprehensive analysis of the final drug product's quality attributes, including purity and potency, confirmed consistency across all batches. In nonclinical studies using cynomolgus macaques, GIGA-2050 was found to be well-tolerated and safe, with a half-life comparable to other recombinant human IgG1 antibodies. Furthermore, in vivo studies in mice demonstrated that GIGA-2050 provided protection against a lethal SARS-CoV-2 infection, resulting in a 60% survival rate. The compelling results from these studies were instrumental in supporting a successful Investigational New Drug (IND) application. This work provides a precedent for the large-scale manufacturing of recombinant polyclonal hyperimmune globulin drugs to treat infectious diseases, highlighting the potential to overcome the limitations of traditional methods.
FAQs
Q: What is the main difference between preclinical and IND-enabling studies?
A: Preclinical studies encompass a broad range of early-stage research to identify a lead drug candidate. IND-enabling studies are the final, more rigorous preclinical studies specifically designed to generate the safety and toxicology data required for a regulatory submission to begin human trials.
Q: How does GMP impact my drug's development timeline?
A: Implementing GMP from the start can significantly accelerate your timeline. It ensures your drug is manufactured to regulatory standards from the beginning, preventing costly delays and the need for re-manufacturing or re-testing later in the process.
Q: Are IND-enabling studies expensive?
A: The cost of IND-enabling studies can vary widely depending on the type of drug, the complexity of the studies, and the animal models required. However, these studies are a necessary investment to de-risk the project and demonstrate safety to regulators, ultimately saving time and resources in the long run.
Q: Can you help with the regulatory filing process for an IND application?
A: While the core of our service is to provide the data and manufactured material for your IND application, our experts can provide guidance on the necessary data packages and requirements to ensure a smooth and successful submission
Q: What if my drug is a biologic? Do you have experience with that?
A: Yes, we have extensive experience with biologics. Our facilities and expertise are specifically tailored to handle the unique challenges of manufacturing complex biological molecules, ensuring their stability, potency, and purity.
Creative Biolabs is a leading provider of integrated IND-Enabling Studies & GMP Manufacturing Support. We offer a holistic solution that combines robust preclinical data generation with high-quality, regulatory-compliant manufacturing. Our proven expertise and innovative platforms are designed to reduce risk and accelerate your drug development timeline, helping you confidently move your therapeutic agent from the lab to the clinic.
Connect with our experts for project-specific consultation and detailed insights.
Reference
- Mizrahi, Rena A et al. "GMP Manufacturing and IND-Enabling Studies of a Recombinant Hyperimmune Globulin Targeting SARS-CoV-2." Pathogens (Basel, Switzerland) vol. 11,7 806. 19 Jul. 2022, Distributed under Open Access license CC BY 4.0, without modification. https://doi.org/10.3390/pathogens11070806



