Biotin based Conjugation Protocol
Background Introduction
Biotin (also known as Vitamin H or B7) is a small, water-soluble vitamin renowned for its exceptionally high affinity (Kd ≈ 10−15 M) for avidin or streptavidin. This non-covalent interaction is one of the strongest known biological interactions and is characterized by its high specificity. Biotin-based conjugation leverages this property to label target molecules (such as antibodies, proteins, nucleic acids, etc.), thereby enabling their detection, isolation, immobilization, or visualization through the biotin-streptavidin interaction.
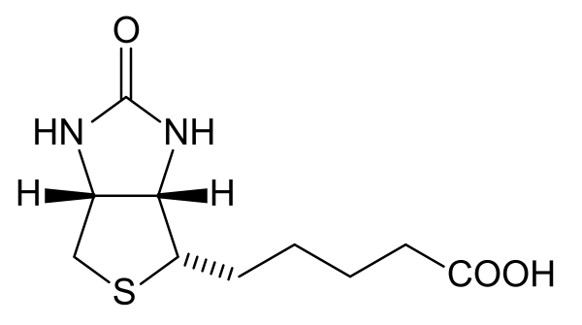 Fig.1 The structure of Biotin.Distributed under public domain, from Wiki, without modification.
Fig.1 The structure of Biotin.Distributed under public domain, from Wiki, without modification.
In biomedical research and diagnostics, biotin-antibody conjugation is an extensively used technique. By labeling antibodies with biotin, researchers can utilize streptavidin-conjugated enzymes (e.g., HRP, AP), fluorophores, or magnetic beads to achieve indirect antibody detection, thereby amplifying signals, enhancing sensitivity, and providing greater experimental flexibility. Compared to direct antibody labeling, biotinylation often better preserves antibody activity and allows for multi-step detection strategies.
This protocol will detail the operational steps and considerations for conjugating N-hydroxysuccinimide (NHS) ester-activated biotin derivatives (long-chain biotin NHS ester, LCB-NHS) to proteins, specifically antibodies. The NHS ester group reacts with primary amine groups (-NH2) on lysine residues of the protein surface, forming a stable amide bond.
Disclaimer
This protocol is intended for research purposes only and is not for diagnostic or therapeutic use. All operations should be conducted in a laboratory environment with appropriate safety precautions. The processes and technologies outlined in this document are for reference only. Creative Biolabs does not guarantee or warrant any specific outcomes from the customer's application of this guideline.
Materials
- Purified monoclonal or polyclonal antibody.
- LCB-NHS (requires dissolution in anhydrous DMSO before use).
- Phosphate-Buffered Saline (PBS, pH 7.2-7.5)
- Quenching Buffer: 1 M Tris-HCl, pH 7.5-8.0.
- Dialysis Buffer: PBS, pH 7.2-7.5, or a buffer suitable for downstream application.
Methods
Antibody Preparation
- Buffer Exchange (if necessary): If the antibody solution contains primary amines (e.g., Tris, glycine) or protein stabilizers (e.g., BSA), perform a buffer exchange into an appropriate amine-free reaction buffer (e.g., PBS, pH 7.2-7.5). This can be done using dialysis (e.g., overnight at 4°C with multiple buffer changes) or desalting columns according to the manufacturer's instructions.
- Concentration Adjustment: Prepare the antibody solution to a final concentration of 2 mg/mL using the reaction buffer.
- Temperature Equilibration: Place the antibody solution on ice or at room temperature, depending on the reaction conditions chosen.
Biotinylation Reaction
- Prepare Biotinylation Reagent Solution: Dissolve LCB-NHS at a concentration of 6 mg/mL (6.5 mM) in anhydrous DMSO before use.
- Incubation: Combine 10 µL of LCB-NHS solution with 1 mL of the antibody solution. The reaction should then proceed at room temperature for 30-60 minutes, or alternatively, at 4°C for a minimum of 2 hours, with continuous gentle agitation.
- Quenching Reaction: Add 0.5 mL of 1 M Tris-HCl (pH 7.5-8.0) to the reaction mixture. Incubate for an additional 15-30 minutes at room temperature to ensure complete quenching.
Purification of Biotinylated Antibody
- Dialysis: Transfer the quenched reaction mixture to a dialysis cassette or tubing with an appropriate molecular weight cut-off (MWCO) (e.g., 10−14 kDa for antibodies). To ensure thorough removal of unreacted biotinylation reagent and quenching buffer components, dialyze the sample against a substantial volume of dialysis buffer (e.g., PBS, pH 7.2-7.5), performing at least 2-3 buffer changes over a 12-24 hour period..
- Desalting Column (Alternative): Alternatively, use a pre-packed desalting column equilibrated with the desired dialysis buffer. Apply the reaction mixture to the column and elute the biotinylated antibody according to the manufacturer's instructions. This method is faster but may result in some sample dilution.
Notes
- Ensure all buffers used are free of primary amines (Tris, glycine, ammonium salts) during the biotinylation reaction, as these will react with the NHS-ester and reduce labeling efficiency.
- NHS-activated biotin effectively reacts with primary amine groups in buffers with pH 7-9 to form stable amide bonds, typically using PBS pH 7.4 or pH 8.0.
- NHS-ester biotinylation reagents are susceptible to hydrolysis in aqueous solutions. Prepare fresh solutions immediately before use.
-
Troubleshooting:
- Low Biotin Incorporation: Check buffer composition (presence of amines), pH, reaction time/temperature, and biotinylation reagent integrity. Increase the molar excess of biotinylation reagent.
- Loss of Antibody Activity: May be due to over-biotinylation (too many biotins per antibody), biotinylation of critical binding sites, or antibody denaturation during the process. Reduce biotinylation ratio, optimize reaction conditions, or ensure gentle handling.
- Aggregation/Precipitation: Can be caused by over-biotinylation, high protein concentration, or unsuitable buffer conditions. Try lower biotinylation ratios, lower protein concentrations, or different reaction buffers.
Creative Biolabs provides professional, comprehensive services for projects involving Biotin Conjugation, ensuring high-quality results. Please contact us for more information.
