Conjugation Based on Click Chemistry
Click chemistry is a remarkable synthetic approach that enables the rapid and efficient construction of novel molecules through the formation of carbon-heteroatom bonds (C-X-C). This versatile method encompasses a range of reactions, including cycloadditions, non-aldol carbonyl chemistry, nucleophilic ring-opening reactions, and additions to carbon-carbon multiple bonds. The advantages of click chemistry are numerous, including bioorthogonality, mild reaction conditions, high yields, stereospecificity, easy separation of products, and the ready availability of starting materials. Consequently, click reactions have emerged as a valuable and straightforward tool for bioconjugation, facilitating a wide range of applications in diverse fields. The methods for preparing antibody-drug conjugate (ADC) using the strain-promoted azide-alkyne cycloaddition (SPAAC) reaction and labeling DNA and oligonucleotides using the copper-catalyzed azide-alkyne cycloaddition (CuAAC) reaction are described below.
Disclaimer
The processes and technologies outlined in this document are for reference only. Creative Biolabs makes no assurances or warranties regarding the outcomes that may result from the customer's implementation of this guideline.
Materials
 ADC Preparation by SPAAC
ADC Preparation by SPAAC
- Azide-conjugated antibody.
- Dibenzoazacyclooctyne (DBCO)-conjugated drug-linker.
- Phosphate buffer saline (PBS, pH 7.4).
- Dimethyl sulfoxide (DMSO).
- Ultracentrifugal filter (50 kDa MWCO).
- Desalting column.
- Syringe filter (0.2 μm).
 DNA and Oligonucleotides Labeling by CuAAC
DNA and Oligonucleotides Labeling by CuAAC
- Alkyne-conjugated oligonucleotides or DNA.
- Copper (II)-tris(benzyltriazolylmethyl)amine (TBTA) complex.
- Azide.
- Ascorbic acid.
- DMSO.
- Triethylammonium acetate buffer (pH 7.0).
- Polyacrylamide gel.
- DNA PAGE gel extraction kit.
Methods
 ADC Preparation by SPAAC
ADC Preparation by SPAAC
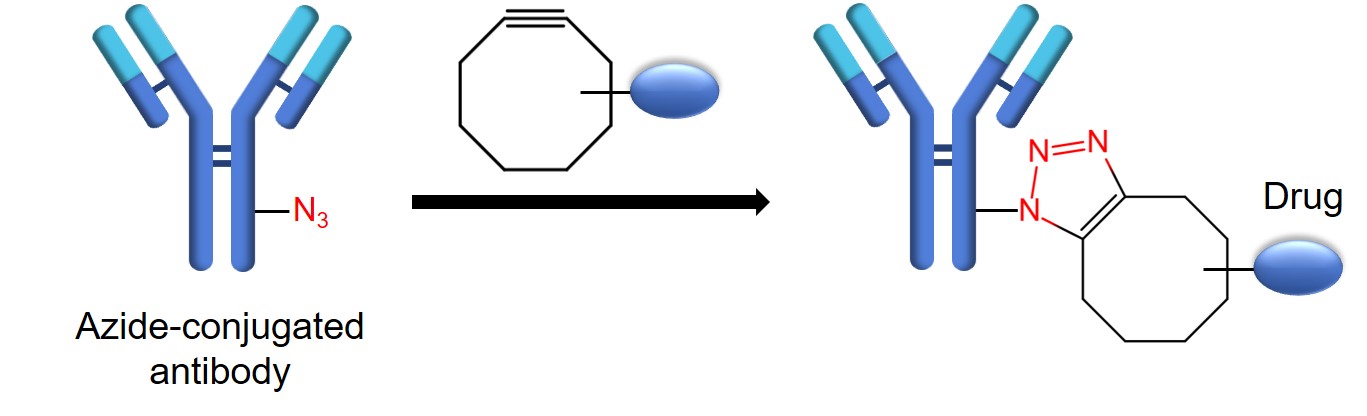
- Take the purified azide-conjugated antibody and perform a buffer exchange using a desalting column equilibrated with PBS (pH 7.4).
- Prepare the stock solution (26.7 mM) of a drug linker conjugated with DBCO in DMSO.
- Add 50 μL of 26.7 mM DBCO-drug linker stock solution and 10 mg of azide-conjugated antibody in PBS (pH 7.4) containing 5% DMSO up to 1 mL final volume of the sample.
- Incubate the samples for 2 hours at room temperature.
- Remove excess DBCO-drug linkers by placing the sample in a desalting column equilibrated with PBS (pH 7.4).
- Use a 50 kDa MWCO protein concentrator to concentrate the obtained ADC.
- Use a syringe filter (0.2 μm) to filter the purified ADC samples.
- Store the final samples at -20 ~ -80°C.
 DNA and Oligonucleotides Labeling by CuAAC
DNA and Oligonucleotides Labeling by CuAAC
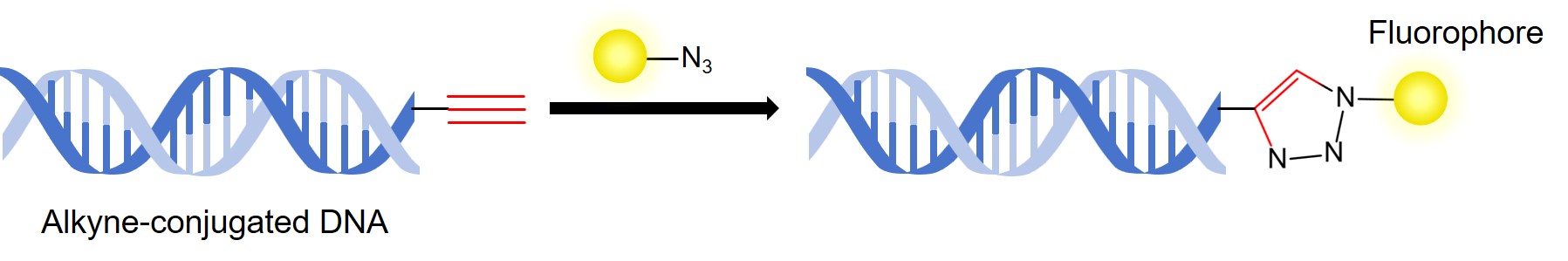
- In a pressure-tight vial, dissolve the alkyne-conjugated DNA or oligonucleotide in water. Afterwards, add triethylammonium acetate buffer (pH 7.0, 2 M) and DMSO, and then vortex.
- Add 10 mM azide stock solution in DMSO to the mixture, and then vortex.
- Add 5 mM ascorbic acid solution in water to the mixture, and then give it a brief vortex.
- Degass the solution by bubbling nitrogen in it for 30 seconds.
- Add the copper (II)-TBTA stock solution (10 mM in 55% DMSO) to the mixture. After pumping nitrogen into the vial, shut the top.
- Vortex the final mixture thoroughly and keep it at room temperature overnight.
- Use at least four times as much ethanol (for DNA) or acetone (for oligonucleotides) to precipitate the conjugate. After fully mixing, store at -20°C for 20 minutes.
- Centrifuge the conjugate for 10 minutes at 10,000 rpm, and then discard the supernatant. After washing the particle with 1 mL of acetone, centrifuge it once again for 10 minutes at 10,000 rpm. Remove the supernatant and dry the conjugate.
- Purify the conjugate by polyacrylamide gel electrophoresis (PAGE), and then recover DNA or oligonucleotides by DNA PAGE gel extraction kit.
Notes
- Besides DMSO, DBCO-drug Linker can be dissolved in dimethylformamide (DMF) or dimethylacetamide (DMA).
- Excess DBCO-drug Linker compared to the antibody and higher reactant concentrations lead to more efficient conjugation.
- The solubility of the drug linker determines the percentage of organic co-solvent. The lower the solubility of the drug linker, the higher the percentage of organic co-solvent required.
- If the conjugation efficiency does not achieve 100%, the ADC samples can be additionally purified with hydrophobic interaction chromatography (HIC).
- Other buffers with a buffer range close to neutral pH can also be used except for azide-containing buffers.
- Other cyclooctyne-conjugated drug-linkers can also be used, such as bicyclononyne (BCN) and dibenzocyclooctyne (DIBO).
- Other inert gases can also be used to degass the solution, such as argon and helium.
- If you notice any noticeable azide precipitation during the final vortex of combination, heat the mixture for 3 minutes at 80°C and vortex again.
Recommended products
