DNA-Encoded Library (DEL) Technology in Drug Discovery
Introduction of DNA-Encoded Library (DEL)
The emergence of DNA-encoded library (DEL) platforms has fundamentally transformed compound screening architectures in contemporary drug discovery pipelines. In contrast to conventional iterative synthesis-test-analyze sequences, this paradigm employs combinatorial synthesis principles to generate molecular diversity across 10⁶–10⁹ unique entities. The methodology's true innovation lies in its hybrid molecular architecture – synthetic small molecules conjugated to amplifiable genetic information carriers. This molecular symbiosis between organic chemistry and molecular biology protocols enables exponential scaling of compound libraries while maintaining deterministic structural tracking. Consequently, DEL technology accelerates the early stages of drug discovery by enabling the rapid identification of promising leads, surpassing the throughput and efficiency of conventional methods.
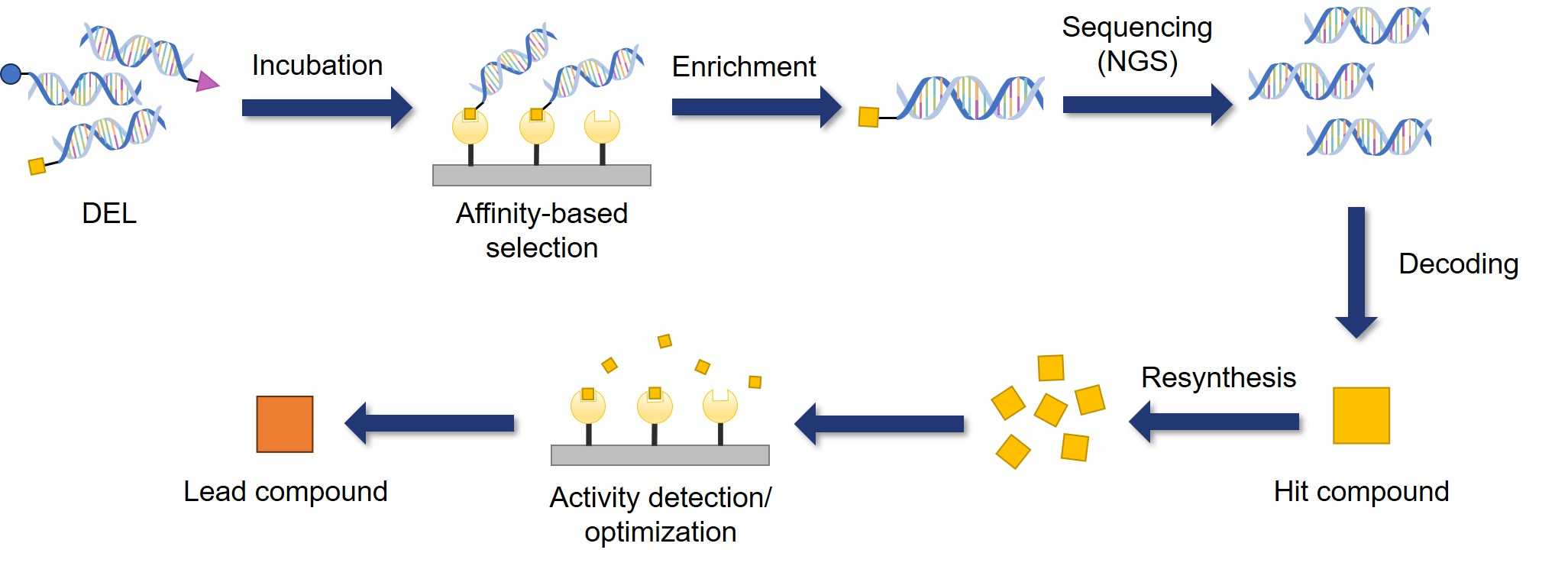 Fig.1 The basic process of DEL screening.
Fig.1 The basic process of DEL screening.
The core of DEL library synthesis is combinatorial chemistry, using the Split & Pool strategy. In this method, DNA-tagged building blocks undergo iterative splitting, reaction, and pooling processes to construct diverse DEL libraries. Each DNA tag uniquely identifies a synthesis step, enabling simultaneous synthesis and screening of millions or billions of different molecules within the pooled mixture. Following affinity-based selection against biological targets, next-generation sequencing decrypts the retained oligonucleotide signatures, correlating binding events with precise chemical structures.
A typical three-cycle library synthesis proceeds as follows: The starting Headpiece (HP), a linker with reactive ends, has a DNA sequence attached at one end. HP is distributed into the wells of a plate (split process). In each well, a unique DNA tag for the first cycle is attached to the HP. Then, a first-cycle building block is added and reacted with the other end of HP. Wells are combined (pool process), completing the first cycle. The process is repeated for the second and third cycles.
Core Application Areas
The application of DEL technology has catalyzed transformative advances across multiple biomedical disciplines, particularly in pharmacological innovation and biological target deconvolution:
- Therapeutic Lead Discovery: Through affinity-based selection workflows, these combinatorial repositories facilitate the identification of high-affinity binders for disease-relevant proteins. Recent oncology-focused campaigns isolated selective inhibitors of KRASG12C oncogenic variants, while neurodegenerative research identified disruptors of tau fibrillization kinetics—advances that may expedite therapies for pancreatic ductal adenocarcinoma and tauopathies.
- Mechanistic Target Elucidation: Beyond conventional drug discovery, DEL technology enables reverse pharmacological interrogation of bioactive compounds with uncharacterized mechanisms. Through competitive affinity selection workflows, researchers can map compound interactomes across proteomic landscapes. This approach recently resolved a decade-old mystery surrounding the anticancer activity of marine-derived alkaloids, revealing unexpected binding to mitochondrial electron transport chain components. Such findings not only clarify drug mechanisms but also highlight novel therapeutic targets in metabolic disorders.
- Functional Proteomic Tool Development: DEL architectures facilitate the creation of precision chemical probes for mechanistic interrogation of biological systems. Through iterative screening against enzyme families or multiprotein complexes, researchers have isolated covalent modifiers of deubiquitinases. These molecular tools enable real-time tracking of post-translational modification cascades and transient protein interaction networks.
Advantages of DEL Technology
DEL technology offers several key advantages over traditional drug discovery methods:
- Increased Speed and Throughput: DELs enable the screening of billions of compounds in a single experiment, significantly accelerating the hit identification process compared to traditional high-throughput screening (HTS).
- Reduced Cost: DEL synthesis and screening can be more cost-effective than synthesizing and screening individual compounds, especially for very large libraries.
- Expanded Chemical Space: DELs can explore a much larger chemical space than traditional compound libraries, increasing the chances of finding novel and effective ligands.
- Small Amount of Protein Required: DEL screening requires only small amounts of the target protein.
Advances in DEL Screening Strategies
DEL screening strategies have evolved significantly, expanding the scope of targets and improving the identification of functional ligands:
- Affinity Screening: The primary method, involves the selection of DEL compounds that bind to a target protein. This can be performed with the target immobilized on a solid support or in solution.
-
Screening Complex Biological Targets: DEL screening has been adapted for use with challenging targets, including:
- Cell lysates: Screening directly in cell extracts.
- Membrane proteins: Identifying ligands for proteins embedded in cell membranes.
- Live cells: Performing selections in living cells to identify cell-permeable ligands.
Future Perspectives
The future development potential of DEL technology is enormous, mainly in the following areas:
- Integration with Artificial Intelligence (AI): AI and machine learning present opportunities to advance DEL methodologies. These computational tools can be employed to refine DEL design, optimize screening protocols, and improve the interpretation of screening datasets. Furthermore, AI-driven predictions of compound activity and selectivity have the potential to expedite the lead optimization process.
- Automation and Miniaturization: Continued advances in automation and miniaturization will increase screening throughput, reduce costs, and enable the screening of even larger and more diverse DELs.
- Expanding Chemical Space: The development of new DNA-compatible chemical reactions will expand the diversity of DELs, allowing for the exploration of novel chemical structures.
