Background Services Overview Published Data Protocols Related Products Q&A Resources
Autoantibodies to complement components are closely related to many autoimmune diseases, and the testing
of these autoantibodies is of great significance for the study of complement-related diseases. Creative
Biolabs offers a sophisticated menu of complement autoantibody tests and has the expertise to
develop custom assays for diagnostic, research and clinical trial use.
Background
Background of Anti-factor H Autoantibody
Factor H (FH) is a main plasmatic regulatory protein of the alternative complement activation pathway.
FH autoantibodies can impair complement regulation, resulting in the occurrence of some diseases. The
anti-FH autoantibodies’ isotype is primarily IgG but exceptional IgA isotype has also been reported. Anti-FH
IgG autoantibodies production causes FH functional defect, leading to complement activation.
Continuous complement activation is associated with the development of atypical hemolytic uremic syndrome (aHUS) and
dense deposit disease (DDD). Recently, the presence of anti-FH IgG has been reported to be associated with
other clinical renal diseases such as C3 glomerulopathies, but their pathogenicity in these conditions remains
to be assessed. Anti-FH autoantibody testing facilitates the correct diagnosis of these diseases as early as
possible, followed by support for appropriate therapy, including plasma exchanges and immunosuppressive treatments.
Anti-FH IgG represents a diagnostic marker, so its titer determination can be used to assess disease
progression and monitor treatment.
Anti-FH Autoantibody Test
Autoantibodies to FH can be detected by ELISA with purified proteins immobilized on the microtiter
plate. As shown in Fig.1, the purified target antigen (FH, ①) is coated in the wells of a microplate
in a direct ELISA. The diluted plasma containing potentially autoantibodies (②) is then separately
added to each well. After incubation and washing, a solution containing labeled anti-human IgG
antibody (③) is added and incubated. After additional washings, bound IgG can be revealed using enzymatic
colored reaction. During this process, all samples are dispensed in duplicate in the coated and in
the non-coated parts of the plate. The standard curve is obtained by serial dilutions of a reference
sample and the titer is calculated according to the standard curve using a linear regression curve and the
standard values.

Fig. 1 The procedure of ELISA.1, 3
Services Overview
Our Services of Anti- FH Autoantibody Test
For many years, Creative Biolabs has provided complement autoantibody tests and contract
research services to pharmaceutical, biotechnology companies and researchers around the world to support
their complement research projects and clinical trials. In anti-FH autoantibody testing, we have a variety of
ELISA assay platforms and techniques, which allow the detection of these autoantibodies to facilitate
customer’s subsequent experimental progress. Based on this, our test menu offers
top-quality anti-FH autoantibody tests and related services to meet the various needs of our customers.
Service highlight:
-
High sensitivity and specificity
-
No significant cross-reactivity or interference
-
Accurate results and report analysis
We will continue to make unremitting efforts to provide services and technology to meet
customer's requirements. Please feel free to contact us for more information
about our complement testing services.
Published Data
Presented are findings showcased within articles pertaining to FH autoantibody test:
1. Anti-FH autoantibody ELISA
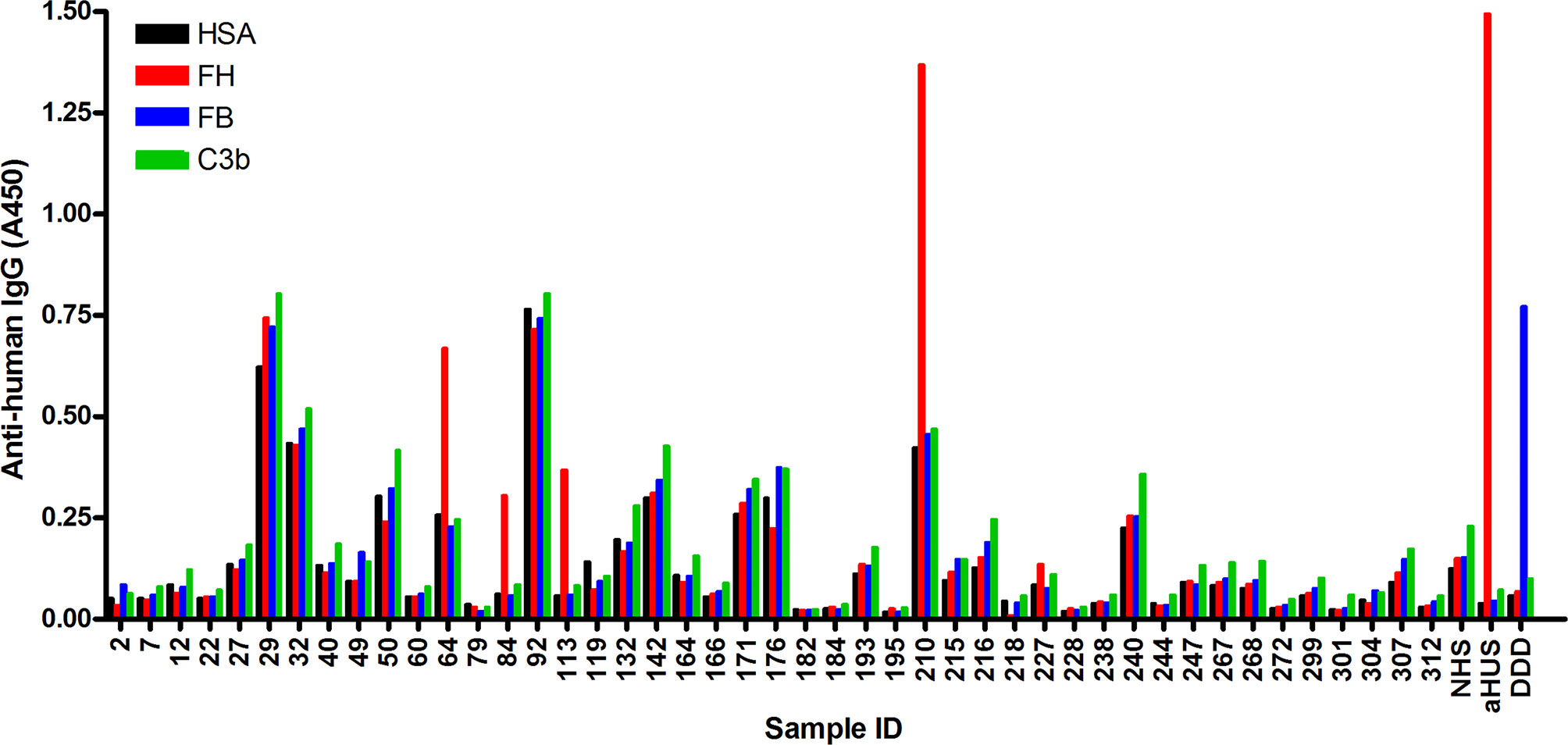
Fig. 2 Screening of NMOSD sera for autoantibodies by ELISA.2, 3
Barbara et al. performed an ELISA to identify autoantibodies to complement proteins in the sera of patients with
NMOSD using immobilized FH, C3b and FB, and solid-phase C3bBb converting enzyme. 4 of 45 samples analyzed were
positive for FH autoantibodies, and no specific autoantibodies against C3b, FB, or C3bBb were detected in any of the
samples. Because FH autoantibodies are strongly associated with deletion of the CFHR1 gene in aHUS, they went on to
investigate the presence of FHR-1 protein in the serum. Both FHR-1 isoforms were detected in the serum by protein
blot analysis.
Protocols
Related Products
Resources
References
-
Boguszewska, Karolina, et al. "immunoassays in DNA damage and instability detection." Cellular and molecular life sciences 76.23 (2019): 4689-4704.
-
Uzonyi, Barbara, et al. "Autoantibodies against the complement regulator factor H in the serum of patients with neuromyelitis optica spectrum disorder." Frontiers in Immunology 12 (2021): 660382.
-
under Open Access license CC BY 4.0, without modification
Questions & Answer
A: Factor H is a member of the regulators-of-complement-activation (RCA) family of proteins, which controls the alternative pathway (AP) of complement in the fluid phase and on cell surfaces. Acquired autoantibodies targeting factor H are called anti-factor H autoantibodies, and they are one of the major factors that impair factor H function. When anti-factor H autoantibodies impair the regulatory function of factor H, complement-mediated tissue damage and inflammation occur.
A: When anti-factor H autoantibodies cause impaired factor H function, it leads to diseases such as atypical hemolytic uremic syndrome (aHUS), C3 glomerulopathy (C3G) and monoclonal gammopathy of renal significance (MGRS). Dysregulation of the alternative pathway controlled by complement factor H plays a key role in the development of aHUS, resulting in the production of anti-factor H autoantibodies. The severity of HUS caused by anti-factor H autoantibodies is much higher than that of other causes of HUS. Apart from aHUS, it has been associated with many other diseases such as age-related macular degeneration, immunoglobulin (Ig) nephropathy.
A: Because many diseases are known to be associated with mutations or dysregulation of factor H, the study of anti-factor H autoantibodies allows the exploration of the molecular mechanisms underlying related autoimmune diseases, which can be applied for diagnosis and therapy development. Many ELISA methods are used to detect anti-factor H autoantibodies. This helps to understand and determine the importance of different anti-factor H autoantibody titers in disease. Detection of anti-factor H autoantibodies at the onset of the disease allows for the application of appropriate therapy.
A: To place an order, simply contact our customer service team or visit our website to submit an order request. Our representatives will guide you through the ordering process and provide any necessary assistance to ensure a smooth and seamless experience.
For Research Use Only.
Related Sections: