C1q Structure C1q Functions C1q-Binding Assays C1q in Disease Therapeutic Target
C1q is the recognition subcomponent of the C1 complex in the classical complement pathway, a crucial part of the innate immune system. It plays a vital role in immune defense by recognizing immune complexes, apoptotic cells, and microbial surfaces, triggering a cascade leading to pathogen elimination.
Structure of C1q
Complement component C1q exhibits a distinctive tulip-like structure composed of six heterotrimeric subunits arranged in a hexameric bouquet. This intricate architecture enables its dual roles in immune recognition and complement activation.
C1q is a 460 kDa glycoprotein organized into:
-
Central fibril-like stalk: Formed by the N-terminal collagen-like regions of six heterotrimers.
-
Peripheral globular heads: Six C-terminal heterotrimeric domains (gC1q) clustered at the distal end.
-
The collagen-like regions diverge at a midpoint "kink," creating six individual stalks that terminate in globular heads.
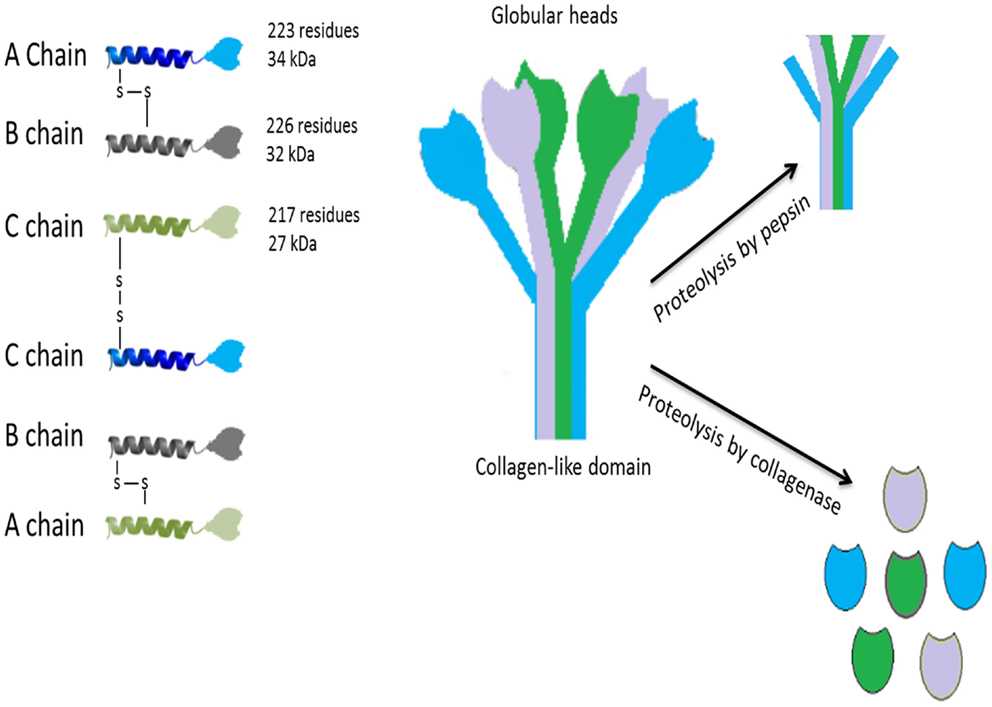 Fig. 1 Structure of C1q.1,3
Fig. 1 Structure of C1q.1,3
Each heterotrimer consists of three chains:
-
A-chain: Contains a collagen-like region and a globular head (ghA).
-
B-chain: Similar structure to A, with globular head (ghB).
-
C-chain: Provides the collagen-like region and globular head (ghC).
The chains are encoded by genes C1QA, C1QB, and C1QC clustered on chromosome 1p36.12 in the order A-C-B.
Each chain contains a Gly-X-Y motif-rich collagen-like region, forming triple helices that stabilize the central stalk. Collagen-like regions provide structural rigidity and multivalent binding capacity and interact with C1r and C1s to form the C1 complex.
The C-terminal globular domains form a heterotrimeric assembly (ghA, ghB, ghC) with a β-sandwich jellyroll topology, structurally homologous to tumor necrosis factor (TNF). Key features:
-
Binding sites: Recognize diverse ligands, including IgG/IgM immune complexes, CRP, and apoptotic cell markers (e.g., phosphatidylserine).
-
Receptor interactions: Engage with gC1qR (CD93) and calreticulin-CD91 to mediate phagocytosis and immune regulation.
-
Crystallographic insights: X-ray structures reveal a clockwise A-B-C arrangement when viewed from the top, critical for ligand recognition.
This modular design underpins C1q's role as a pattern recognition molecule and its involvement in both complement activation and non-complement immune functions.
Functional Roles of C1q
C1q is a multifunctional protein with complement-dependent and independent roles that span immune defense, tissue homeostasis, and disease modulation. Its diverse functions are underpinned by its ability to recognize pathogens, regulate immune cells, and interact with extracellular ligands.
Table 1 Diverse functions of C1q.
|
Functional Roles
|
Mechanisms
|
Key Aspects
|
|
Classical Pathway Activation
|
C1q initiates the classical cascade by binding antigen-antibody complexes (immune complexes) via its globular heads. This interaction activates C1r and C1s, leading to downstream cleavage of C4 and C2, forming the C4b2a convertase.
|
-
IgG/IgM recognition: Binds IgG1, IgG2, IgG3, and IgM, but not IgG4.
-
Pathogen clearance: Activates complement on bacterial/viral surfaces, enhancing phagocytosis and lysis.
-
Immune complex solubilization: Prevents deposition in tissues by tagging complexes for clearance.
|
|
Pattern Recognition and Pathogen Defense
|
C1q acts as a pattern recognition molecule (PRM), binding diverse ligands.
|
-
Pathogens: Recognizes bacterial porins, LPS, and viral proteins (e.g., HIV-1, HTLV-1).
-
Apoptotic cells: Binds phosphatidylserine and DNA (via deoxy-d-ribose moieties) to mediate clearance without inflammation.
-
Self-molecules: Interacts with CRP, pentraxin 3, and extracellular matrix proteins (e.g., laminin, fibronectin).
-
Competitive binding: DNA and heparin compete for C1q binding but are poor complement activators compared to immune complexes.
|
|
Immune Regulation and Autoimmunity Prevention
|
C1q maintains immune tolerance and prevents autoimmune diseases like systemic lupus erythematosus (SLE).
|
-
Apoptotic cell clearance: Deficiency leads to immune complex deposition and SLE.
-
T-cell/B-cell modulation: Regulates T-cell proliferation, IFN-γ production, and B-cell tolerance checkpoints.
-
Anti-inflammatory signaling: Inhibits excessive inflammation via receptor interactions (e.g., C1qR1, calreticulin-CD91).
|
|
Non-complement Functions
|
Beyond complement activation, C1q influences tissue homeostasis and disease progression.
|
-
Angiogenesis: Promotes endothelial cell permeability, proliferation, and migration.
-
Neuroprotection: Maintains synaptic plasticity and prevents neuroinflammation.
-
Tumor microenvironment: Enhances cancer progression by modulating immune cell infiltration and angiogenesis.
-
Pregnancy: Supports spiral artery remodeling and trophoblast-endometrial interactions.
|
Receptor-mediated Signaling
C1q interacts with cell surface receptors to modulate immune responses:
-
C1qR1 (CD93): Mediates phagocytosis and endothelial cell signaling.
-
Calreticulin-CD91: Enhances apoptotic cell clearance.
-
Integrin α2β1: Regulates immune cell adhesion and migration.
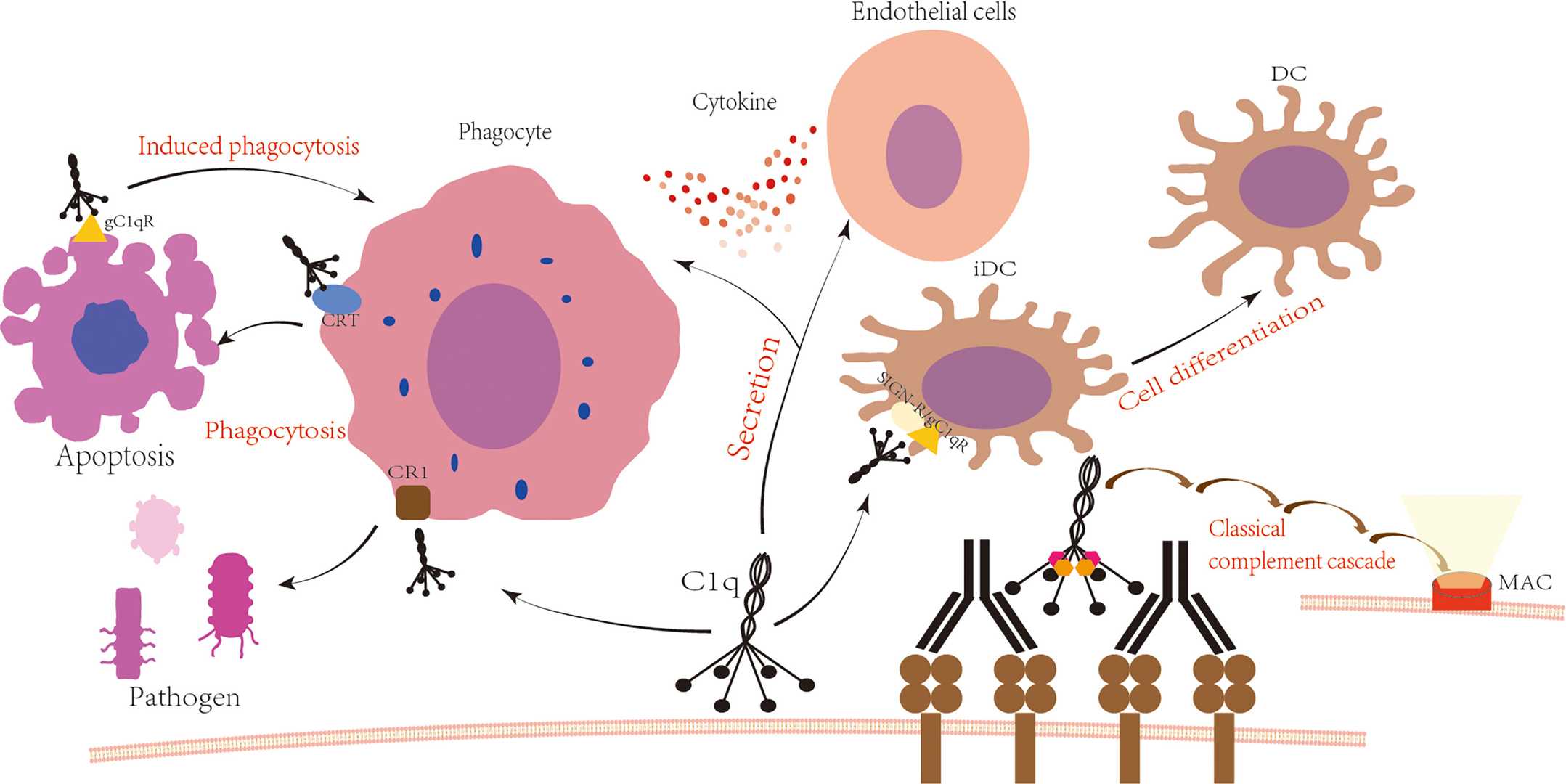 Fig. 2 C1q is involved in the immune process.2,3
Fig. 2 C1q is involved in the immune process.2,3
C1q binding tests are critical tools for evaluating antibody interactions with the complement system, particularly in research and therapeutic development. These assays measure the ability of antibodies (e.g., IgG) to recruit C1q, a key step in complement-dependent cytotoxicity (CDC) and immune complex clearance.
Complement C1q-binding assay is mainly used for measuring the ability of antibody candidates binding to the complement C1q. For the assay, antibody samples developed by clients with different concentration gradients will be tested, and proper positive and negative controls will be included.
During the tests, the binding of test articles to the secondary antibody will be verified first. Then, detection of the binding of samples with different concentration gradients to complement C1q by ELISA titration.
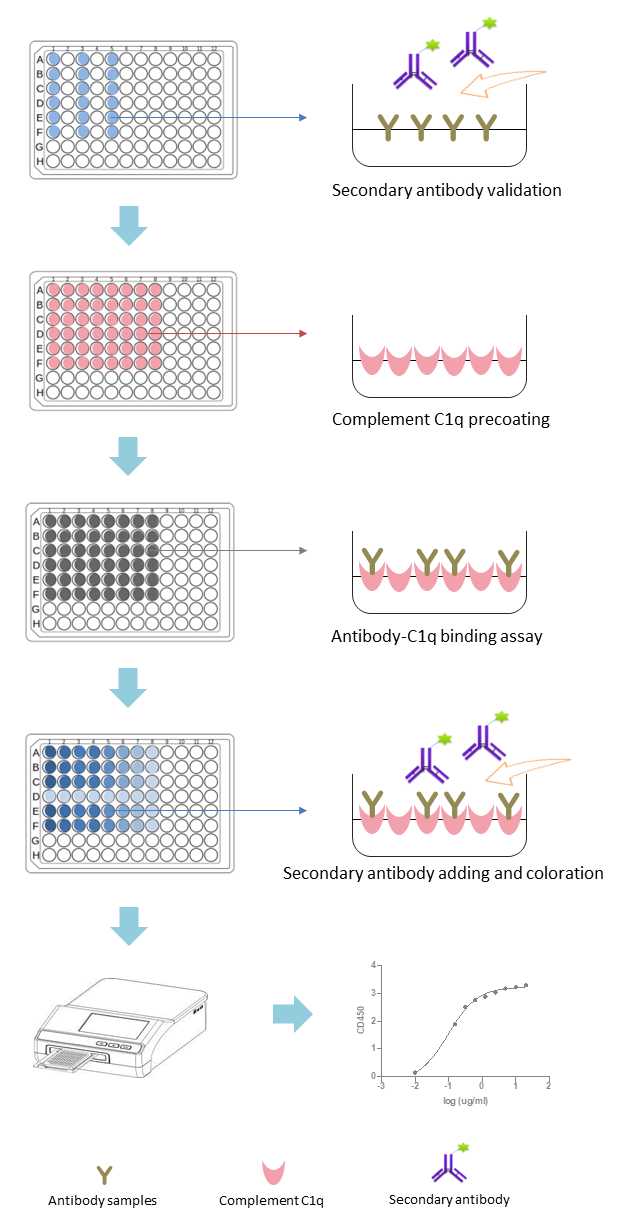 Fig.3 Workflow of complement C1q-binding assays.
Fig.3 Workflow of complement C1q-binding assays.
C1q in Disease and Immunity
C1q plays critical roles in immune regulation, disease prevention, and tissue homeostasis. Its dysregulation or deficiency is implicated in autoimmune disorders, infections, and neurological diseases, underscoring its dual role as both a protective and pathogenic factor.
C1q deficiency is strongly linked to SLE due to:
-
Impaired immune complex clearance
-
T-cell and B-cell dysregulation
C1q deficiency increases susceptibility to encapsulated bacterial infections (e.g., Streptococcus pneumoniae) due to:
-
Impaired opsonization: Reduced phagocytosis of pathogens via complement-dependent mechanisms.
-
Defective immune complex solubilization: Aggregates persist, causing tissue damage.
C1q's role in the CNS is context-dependent.
-
Synaptic pruning: Maintains neuronal network homeostasis during development.
-
Pathogen clearance: Protects against bacterial meningitis and fungal infections.
-
Alzheimer's disease: Amyloid-β binding activates the classical pathway, exacerbating neuroinflammation.
-
Parkinson's disease: Microglial phagocytosis of neuromelanin debris.
-
Prion diseases: Facilitates prion transmission to the CNS in early disease stages.
Table 2 Key mechanisms linking C1q to disease
|
Mechanism
|
Disease Implication
|
|
Apoptotic cell clearance
|
Autoimmunity (SLE), neurodegeneration
|
|
LAIR-1 interaction
|
Suppression of proinflammatory cytokines
|
|
Immune complex deposition
|
Glomerulonephritis, vasculitis
|
|
T-cell dysregulation
|
SLE, chronic infections
|
|
CNS synaptic pruning
|
Neurodevelopmental disorders, neurodegeneration
|
C1q's dual role as a guardian of immune homeostasis and a contributor to disease highlights its importance in both innate immunity and tissue repair. Dysregulation of its functions underscores its potential as a therapeutic target for autoimmune, infectious, and neurological disorders.
C1q as a Therapeutic Target
C1q's dual role in immune regulation and disease pathogenesis has identified multiple therapeutic targets, spanning replacement therapies, receptor modulation, and pathway inhibition.
-
C1q replacement therapy
Fresh frozen plasma (FFP): Provides functional C1q to restore immune complex clearance and suppress autoimmunity.
-
Clinical evidence: Resolves severe cutaneous lesions and reduces SLE flares in C1q-deficient patients.
-
Limitations: Risk of transfusion-related complications (e.g., infections, GVHD).
Recombinant C1q
-
Potential: Engineered C1q variants with enhanced stability or targeted delivery (e.g., gene therapy).
-
Research: Experimental models show restored complement function in C1q-deficient mice.
-
Targeting C1q receptors
Anti-inflammatory target - gC1qR (CD93)
-
Mechanism: Binds C1q's globular heads to suppress pro-inflammatory cytokines (e.g., TNF-α, IL-6) and promote tolerogenic DCs.
-
Therapeutic potential: Agonists mimicking gC1qR binding could enhance immune tolerance in autoimmune diseases.
Pro-inflammatory target - cC1qR
-
Mechanism: Engages C1q's collagen tail to drive DC maturation and Th1/Th17 responses.
-
Therapeutic potential: Inhibitors could reduce inflammation in autoimmune or infectious contexts.
-
Modulating C1q's anti-inflammatory functions
-
Enhancing tolerogenic signaling
-
Suppressing IFN-α production
-
Inhibiting C1q in neuroinflammation
-
Gene therapy for C1q deficiency: Restoring C1q production in monocytes via gene editing (e.g., CRISPR-Cas9).
As a key player in complement biology, C1q remains an important focus for advancing immunologic diagnosis and therapy.
Creative Biolabs is a global leader in biotechnology solutions, specializing in antibody development, protein engineering and therapeutic discovery. For customized solutions for complement system research, please contact our team of experts.
References
-
Kouser, Lubna, et al. "Emerging and novel functions of complement protein C1q." Frontiers in immunology 6 (2015): 317. https://doi.org/10.3389/fimmu.2015.00317
-
Zhang, Wenjie, Yuan Chen, and Hui Pei. "C1q and central nervous system disorders." Frontiers in Immunology 14 (2023): 1145649. https://doi.org/10.3389/fimmu.2023.1145649
-
under Open Access license CC BY 4.0, without modification.
For Research Use Only.
Related Sections:

 Fig. 1 Structure of C1q.1,3
Fig. 1 Structure of C1q.1,3
 Fig. 2 C1q is involved in the immune process.2,3
Fig. 2 C1q is involved in the immune process.2,3
 Fig.3 Workflow of complement C1q-binding assays.
Fig.3 Workflow of complement C1q-binding assays.