C1r Structure C1r Functions C1r Assays C1r in Disease Research Directions
Complement component C1r is a serine protease that plays a pivotal role in the classical pathway of the complement system. As a modular enzyme, C1r mediates the activation of the C1 complex, initiating a cascade that eliminates pathogens and damaged cells.
Structure of C1r
C1r exhibits a modular design that enables its dual role in proteolytic activation and structural organization within the C1 complex. Its architecture comprises multiple functional domains arranged in a specific sequence, facilitating interactions with other complement components and precise enzymatic activity.
From N-terminal to C-terminal, C1r contains:
-
CUB modules: Two CUB domains (CUB1 and CUB2) mediate interactions with C1s and C1q. These modules are critical for maintaining the integrity of the C1 complex and enabling Ca²⁺-dependent binding to collagenous regions of C1q.
-
Epidermal Growth Factor (EGF)-like module: This domain contributes to structural stability and facilitates calcium-dependent interactions, particularly during the formation of the C1r–C1s heterodimer.
-
Complement Control Protein (CCP) modules: Two contiguous CCP domains (CCP1 and CCP2) form part of the catalytic region, enabling homodimerization and interactions with the serine protease (SP) domain.
-
SP domain: The catalytic core responsible for autolytic activation and cleavage of C1s. This domain adopts a chymotrypsin-like fold, with a disulfide bond linking CCP1 to the SP domain, influencing conformational changes during activation.
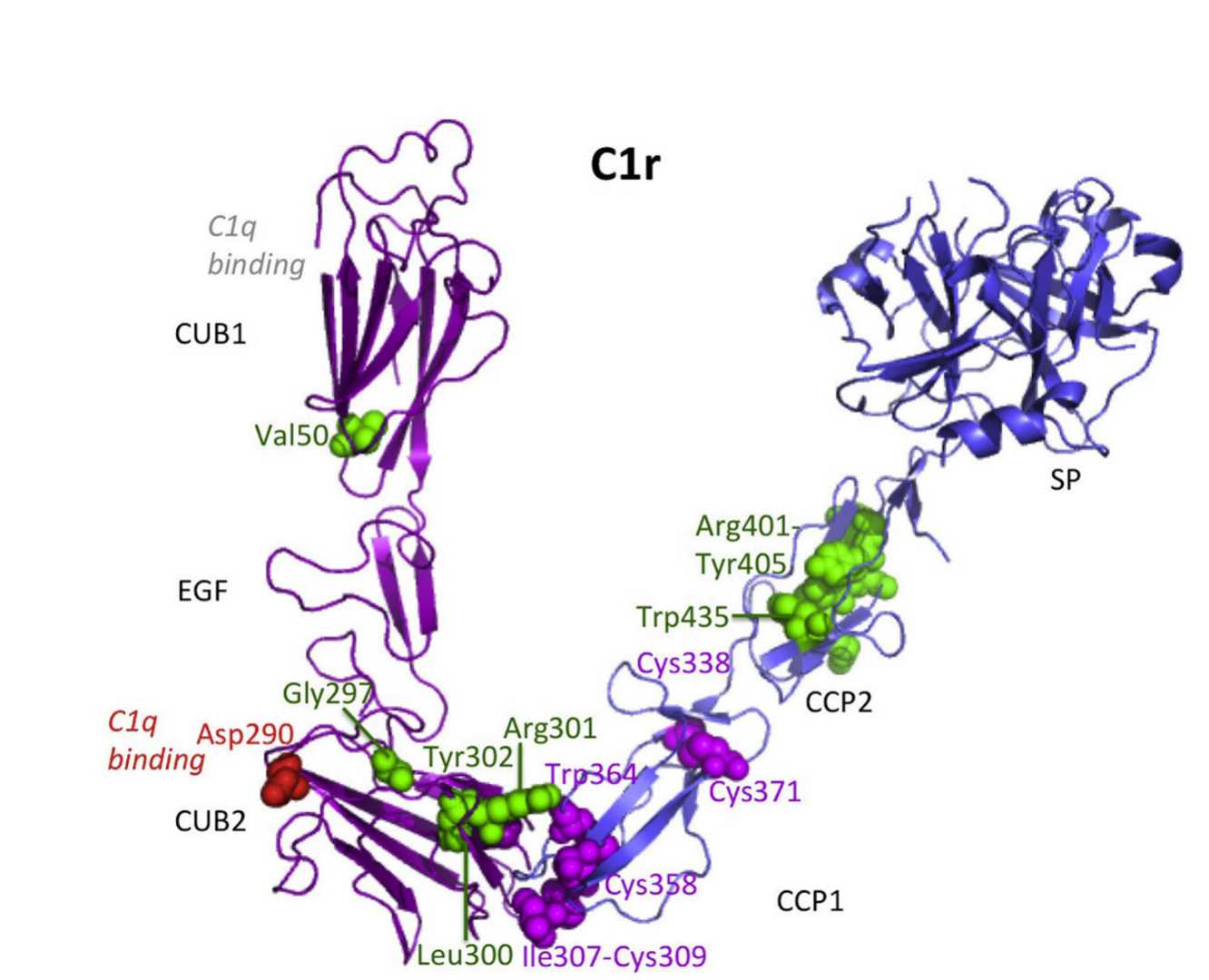 Fig. 1 Structure of C1r.1,2
Fig. 1 Structure of C1r.1,2
The catalytic region of C1r (CCP1-CCP2-SP) forms a homodimeric structure through head-to-tail interactions. This arrangement positions the catalytic site of one monomer opposite the cleavage site of the other, ensuring precise coordination during activation. The SP domains are located at the periphery of the C1 complex, enabling intermolecular proteolytic activation between neighboring C1 complexes bound to a pathogen surface.
C1r and C1s form a compact heterodimer via their N-terminal CUB1-EGF-CUB2 domains. This interaction is stabilized by Ca2⁺ions and involves extensive contacts spanning all three domains of each protease. The L-shaped fragments interlock in an antiparallel arrangement, creating a larger interface than homodimers, which explains the preferential formation of heterodimers over homodimers. This structural organization ensures efficient activation of the classical pathway by positioning C1r's catalytic domain for cleavage of C1s.
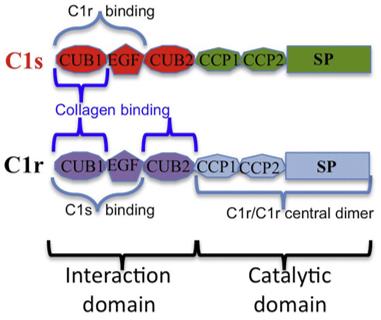 Fig. 2 Modular structure of C1r and C1s and main binding sites to assemble C1.1,2
Fig. 2 Modular structure of C1r and C1s and main binding sites to assemble C1.1,2
Key structural features:
-
Ca²⁺-dependent binding: Critical for maintaining the C1r-C1s interaction and enabling proteolytic activity.
-
Flexibility at the EGF-CUB2 junction: Allows conformational adjustments during activation, as observed in structural studies.
-
Disulfide bond in the SP domain: Stabilizes the catalytic region and influences activation dynamics.
These structural adaptations enable C1r to perform its dual role in autolytic activation and substrate cleavage, making it indispensable for initiating the classical complement cascade.
Functional Roles of C1r in the Classical Pathway
C1r is indispensable for initiating and propagating the classical pathway. Its enzymatic activity and structural interactions drive a cascade that eliminates pathogens, clears cellular debris, and modulates inflammation.
Table 1 Diverse functions of C1r.
|
Functional Roles
|
Key Aspects
|
|
Activation Mechanism
|
-
C1q binding and conformational change: C1q binding induces a conformational change in C1q's collagenous stalks, destabilizing the C1 complex (C1q-C1r2-C1s2) and enabling C1r autoactivation.
-
Autolytic activation of C1r: C1r undergoes self-cleavage at its SP domain, converting from an inactive zymogen to an active protease.
-
C1s activation: Activated C1r proteolytically cleaves C1s, generating an active serine protease. The cleavage occurs at Arg438-Ile439, releasing the enzymatic activity of C1s.
|
|
Propagation of the Complement Cascade
|
-
C4 and C2 cleavage: Activated C1s cleaves C4 into C4b and C4a, followed by C2 into C2a and C2b.
-
C3 convertase form: The C4b-C2a complex forms the C3 convertase (C4b2a), which amplifies the cascade by cleaving C3 into C3b and C3a.
-
Pathogen opsonization and lysis: C3b coats pathogens, marking them for phagocytosis. C5a and C3a act as anaphylatoxins, recruiting immune cells and inducing inflammation. Terminal components (C5-C9) form the membrane attack complex (MAC), lysing pathogens.
|
C1r is a linchpin in the classical pathway, orchestrating proteolytic activation and downstream effector functions. Its structural adaptability, regulatory interactions, and role in pathogen clearance underscore its importance in immune defense and disease pathology.
C1r Assays
Detecting C1r involves both quantitative measurement of its protein levels and functional assessment of its enzymatic activity.
Immunological Assays
-
ELISA
-
Commercial kits: Uses a sandwich enzyme immunoassay technique to quantify C1r in serum or plasma, with results in <4 hours.
-
Advantages: Offers high sensitivity and a broad dynamic range, suitable for serum and plasma samples.
-
Limitations: Detects total C1r protein (active and inactive forms) rather than enzymatic activity.
-
Western Blot
-
Antibody-specific detection: The antibody specifically recognizes the processed, active form of C1r (Ser18–Asp705), distinguishing it from the precursor.
-
Immuno-PCR
-
Immuno-PCR kit: Combines antibody-based capture with PCR amplification for ultrasensitive detection, enhancing signal-to-noise ratios compared to traditional ELISA.
Functional Assays
-
Cleavage activity
-
C4/C2 cleavage: C1r's proteolytic activity can be indirectly measured by detecting cleavage products (C4b, C2a) via ELISA or Western blot.
-
Synthetic substrates: Chromogenic substrates (e.g., S-2314) are used to quantify active C1r, though specificity is limited due to cross-reactivity with other proteases (e.g., C1r, granzyme H).
-
Hemolysis assays
-
CH50: Measures total classical pathway activity in serum, but does not isolate C1r's role.
Table 2 Summary of key assays.
|
Method
|
Application
|
Strengths
|
Limitations
|
|
ELISA
|
Quantify total C1r protein
|
-
High throughput
-
Commercial kits
|
-
Does not measure activity
|
|
Western Blot
|
Detect active C1r (processed form)
|
-
Specificity with antibody
|
-
Labor-intensive
-
Low throughput
|
|
Immuno-PCR
|
Ultrasensitive detection
|
|
-
Requires specialized equipment
|
|
C4/C2 Cleavage
|
Measure enzymatic activity
|
|
-
Cross-reactivity with other proteases
|
Researchers can leverage C1r assays to study complement-mediated diseases and develop targeted therapies.
C1r in Disease and Immunity
C1r plays a dual role in immune defense and disease pathology, with its dysregulation linked to autoimmune disorders, infections, and cancer.
Table 3 Key mechanisms linking C1r to disease
|
|
Disease
|
Mechanism
|
|
Autoimmune Diseases
|
Systemic Lupus Erythematosus (SLE)
|
-
Deficiency link: C1r/C1s deficiencies are strongly associated with SLE-like symptoms, recurrent infections, and elevated levels of C4, C2, and C1-inhibitor (C1-INH).
-
Auto-antibody activation: In SLE patients, elevated C1s levels may reflect auto-antibody-driven C1 activation, though anti-C1s antibodies are less common than anti-C1q antibodies.
-
Lupus nephritis: C1r/C1s-deficient patients often develop severe cutaneous lesions and lupus nephritis due to impaired immune complex clearance.
|
|
Rheumatoid Arthritis (RA)
|
-
C1s activation: Activated C1s is localized to degraded osteoarthritic cartilage, contributing to inflammation and tissue destruction. TNF-α amplifies C1s production in chondrocytes, exacerbating joint damage.
|
|
Infectious Diseases
|
Bacterial Infections
|
-
Susceptibility: C1r/C1s deficiencies increase vulnerability to encapsulated bacteria (e.g., pneumococcal otitis media).
-
Complement activation: C1r/C1s-mediated classical pathway activation is critical for bacterial opsonization and phagocytosis.
|
|
Viral Infections
|
-
COVID-19 severity: Sustained classical pathway activation (involving C1r/C1s) correlates with disease severity, and C1INH therapy reduces inflammation in COVID-19 patients.
|
|
Cancer and Tissue Pathology
|
Tumor Progression
|
-
Tumor-derived C1r/C1s promotes progression by creating an immunosuppressive microenvironment and modulating complement activation.
-
C1r/C1s assembly in tumors may suppress anti-tumor immunity by altering macrophage polarization and dendritic cell function.
|
|
Age-Related Macular Degeneration (AMD)
|
-
Elevated C1s gene expression correlates with AMD progression, suggesting a role in immune cell infiltration and tissue damage.
|
|
Genetic Disorders
|
Ehlers-Danlos Syndrome (Periodontal Type)
|
-
Mutations in the C1R gene are linked to this disorder, characterized by connective tissue fragility and periodontal defects.
|
C1r emerges as a versatile therapeutic target for autoimmune, infectious, and neoplastic diseases, with ongoing research poised to unlock its clinical potential.
C1r Research Directions
C1r remains a focal point for advancing immunological and therapeutic research, with emerging opportunities and challenges shaping its future trajectory. Below are key areas of exploration.
Structural dynamics and activation mechanisms
-
Investigating how C1r's modular domains (CUB, EGF-like, CCP, SP) enable rapid monomer exchange between dimers. This could inform drug design targeting transient activation states.
-
Building on mutant QI (Arg463→Gln), which stabilizes the inactive zymogen form, to study C1r's autoactivation kinetics and intermolecular interactions.
Biomarker development and validation
-
Developing antibodies or biosensors to detect activated C1r (e.g., cleavage-specific epitopes) rather than total protein levels.
-
Establishing reproducible protocols for C1r measurement across diverse populations.
-
Combining C1r with other complement components (e.g., C1s, C1q) or inflammatory markers to enhance diagnostic accuracy.
Therapeutic targeting
-
Designing small-molecule or peptide inhibitors that specifically block C1r's proteolytic activity without broadly suppressing complement function.
-
Expanding on anti-C1s approaches to include C1r-targeted antibodies.
-
Exploring C1r's role in tumor immune evasion and testing its inhibition to enhance anti-tumor immunity.
Complement component C1r is a master regulator of the classical complement pathway, combining structural modularity with precise enzymatic activity. Its role in immune defense and disease pathology underscores the need for continued research into its mechanisms and therapeutic potential.
For customized solutions for complement system research, please contact our team of experts.
References
-
Kapferer-Seebacher, Ines, et al. "Periodontal Ehlers-Danlos syndrome is caused by mutations in C1R and C1S, which encode subcomponents C1r and C1s of complement." The American Journal of Human Genetics 99.5 (2016): 1005-1014. https://doi.org/10.1016/j.ajhg.2016.08.019
-
under Open Access license CC BY 4.0, without modification.
For Research Use Only.
Related Sections:

 Fig. 1 Structure of C1r.1,2
Fig. 1 Structure of C1r.1,2
 Fig. 2 Modular structure of C1r and C1s and main binding sites to assemble C1.1,2
Fig. 2 Modular structure of C1r and C1s and main binding sites to assemble C1.1,2