CD40L loaded Oncolytic Adenovirus Engineering Service
Introduction
For those facing challenges in developing targeted therapies for refractory solid tumors or seeking strategies to enhance anti-tumor immunity and overcome treatment resistance, our OncoVirapy™ Platform offers advanced vector design and immune profiling to engineer potent immunotherapies and accelerate preclinical validation.
[Discover How We Can Help - Request a Consultation]
CD40L-loaded Oncolytic Adenovirus
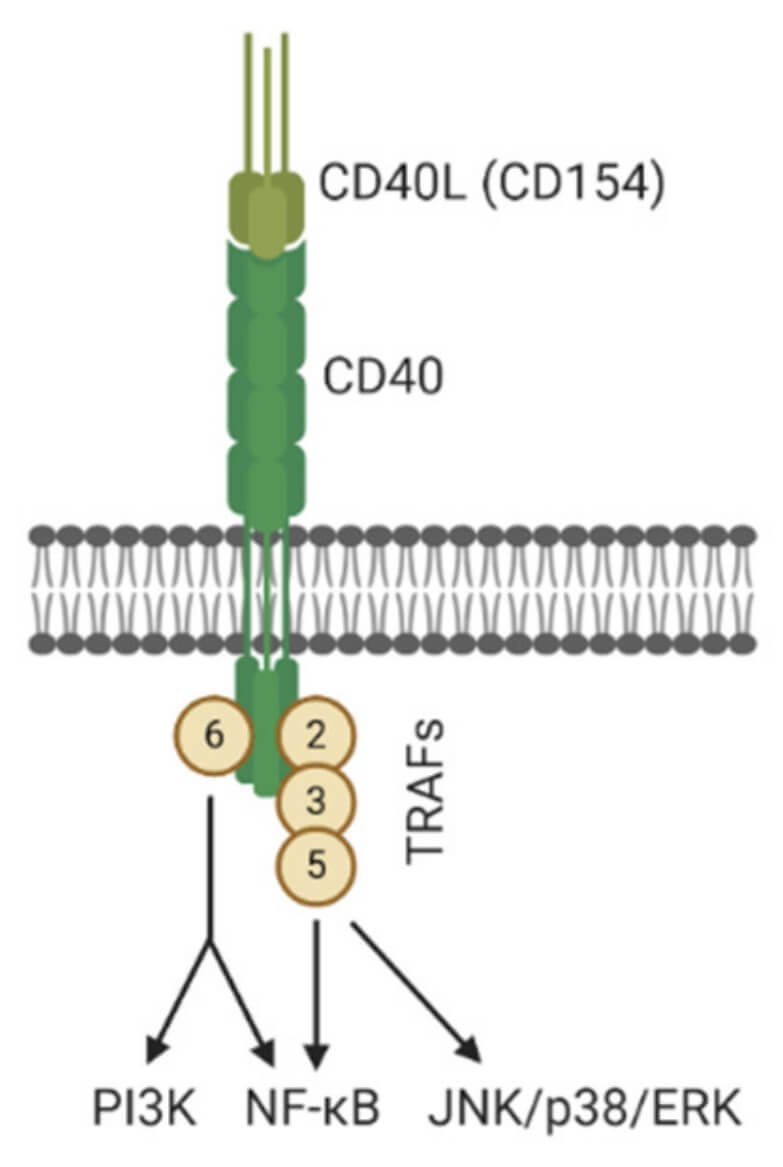 Fig.1 CD40 he CD40L.1
Fig.1 CD40 he CD40L.1
CD40 Ligand (CD40L/CD154) is a key cell surface protein expressed on activated T helper cells and other immune cells. It binds CD40 receptors on antigen-presenting cells (APCs, e.g., B cells, macrophages, dendritic cells) and non-immune cells (endothelial/tumor cells), triggering the CD40/CD40L signaling pathway. This pathway is central to adaptive/innate immune responses, driving APC maturation, cytokine production, and T cell activation/memory formation. Its critical role in immune regulation makes CD40/CD40L a prime therapeutic target for diseases like cancer and chronic inflammation.
Principle
The oncolytic mechanisms of CD40L-loaded oncolytic adenoviruses can be categorized as follows:
1. Direct Oncolysis by Viral Replication
- The oncolytic adenovirus selectively infects and replicates within cancer cells, inducing cell lysis.
- Tumor cell lysis releases tumor-associated antigens (TAAs), providing targets for immune activation.
2. Immunostimulation via CD40L Expression
- The viral vector carries the CD40L gene, which is expressed in infected tumor cells and the TME.
- Local CD40L expression activates antigen-presenting cells, enabling efficient processing and presentation of TAAs to prime and expand CTLs.
3. Remodeling of the Tumor Microenvironment (TME)
- CD40L directly modulates immune cells and stromal components, shifting the immunosuppressive TME to a pro-inflammatory, anti-tumor phenotype.
- This promotes T cell activation, memory formation, and systemic anti-tumor immunity.
Advantages
- Enhanced Anti-Tumor Immunity: CD40L expression in tumors activates APCs to prime/expand tumor-specific T cells, driving durable anti-cancer responses.
- Tumor Microenvironment (TME) Modulation: CD40L reprograms the TME from immunosuppressive (MDSCs/Tregs) to immunostimulatory, promoting anti-tumor cell infiltration and reducing pro-tumor factors.
- Synergistic Combination Potential: Immune-activating CD40L-loaded oncolytic adenoviruses synergize with checkpoint inhibitors, chemotherapy, and targeted agents for enhanced efficacy.
- Targeted Delivery: Oncolytic viruses' tumor selectivity ensures localized CD40L delivery, minimizing systemic toxicity and maximizing therapeutic impact.
Workflow
| Required Starting Materials | Virus Design and Engineering |
|---|---|
|
Our team designs oncolytic adenovirus backbones, inserting the CD40L gene and other therapeutic transgenes (e.g., decorin, IFNβ). We optimize viral replication, tumor tropism, and transgene expression for potent, safe therapy, delivering a validated viral construct ready for production. |
| In Vitro Validation and Characterization | In Vivo Efficacy Studies |
| In vitro studies were performed to confirm oncolytic activity of the engineered virus against target cancer cell lines, to assess CD40L expression and function, and to determine viral replication kinetics | Engineered oncolytic adenoviruses are evaluated in relevant preclinical animal models. Further convincing evidence is provided by evaluating tumor growth inhibition, reduced metastasis, and overall survival. |
| Comprehensive Immune Profiling | Data Analysis and Strategic Recommendations |
| After treatment, systemic and local immune responses were analyzed in detail. Experiments such as flow cytometry and cytokine analysis are used to elucidate the immunomodulatory effects, which can provide insight into the effects of viruses on the host immune system. | Data from in vitro/in vivo studies (efficacy, immune profiling) undergoes rigorous analysis. Our team provides comprehensive interpretations, key insights, and strategic recommendations for development, including combination therapies and optimization strategies. |
| Final Deliverables | Estimated Timeframe |
|
The typical timeframe for this service ranges from 10 to 15 weeks, depending on the complexity of the viral construct, the inclusion of optional preclinical evaluation, and the specific requirements of your project. More intricate designs or extensive in vivo studies may extend the duration. |
What We Can Offer
Creative Biolabs offers comprehensive, customized services for the development and validation of CD40L-loaded Oncolytic Adenoviruses, designed to meet the precise needs of your project. As biology experts, we understand the nuances of therapeutic development and provide end-to-end support, ensuring robust scientific outcomes.
Our Advantage
Custom Viral Engineering and Design
Tailored design of CD40L-loaded oncolytic adenoviruses, optimizing tumor tropism, replication, and co-expression of transgenes per research needs.
Integrated Preclinical Validation
One-stop service including in vitro characterization (oncolysis, immune activation) and in vivo efficacy studies in cancer models for proof-of-concept data.
Advanced Immune Profiling
Analysis of systemic/local immune responses via immune cell quantification (CD8+ T cells, MDSCs, Tregs) and cytokine profiling to clarify immunomodulatory mechanisms.
Strategic Consultation and Data Interpretation
Expert analysis of data by biology specialists, with recommendations to optimize therapeutic development and facilitate clinical translation.
Unwavering Quality and Reproducibility
Rigorous quality control across viral construction, data collection, and analysis to ensure scientific integrity and reproducibility.
Scalable Solutions
Capabilities for projects of all scales, from small in vitro studies to large in vivo evaluations of efficacy and combination therapies.
Case Study
The utilization of CD40L-loaded oncolytic adenoviruses in preclinical models and tumor cell lines enhances tumor-specific lytic activity. Studies validate its potential for solid tumors, highlighting the virus's dual-mechanism strategy.
| Oncolytic Virus Construction | Expression of GM-CSF |
|---|---|
|
|
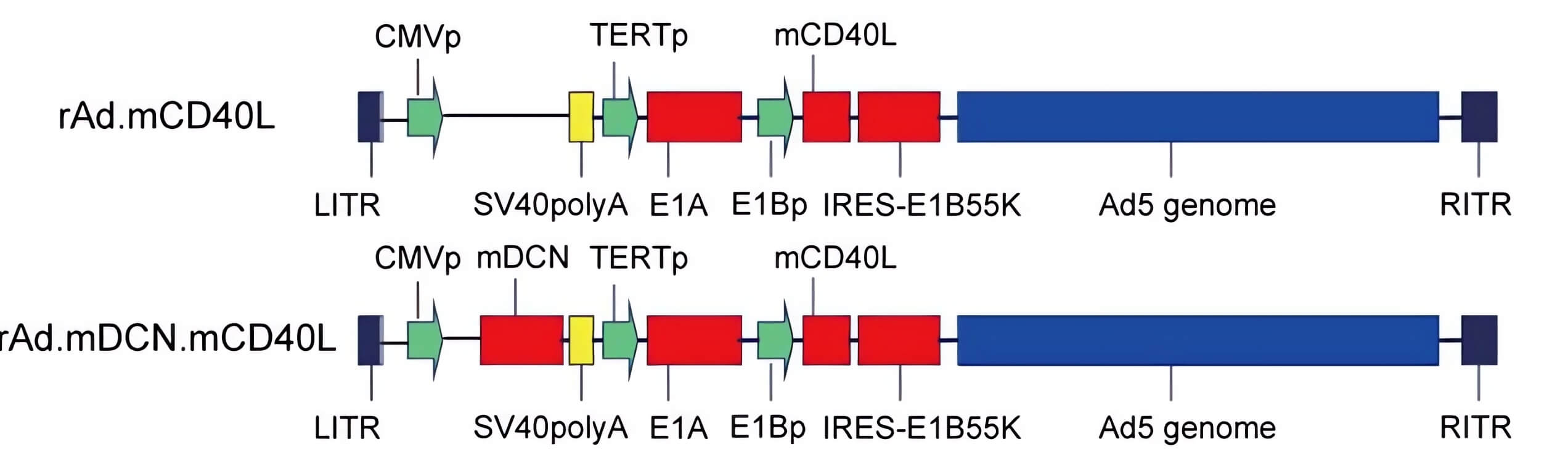
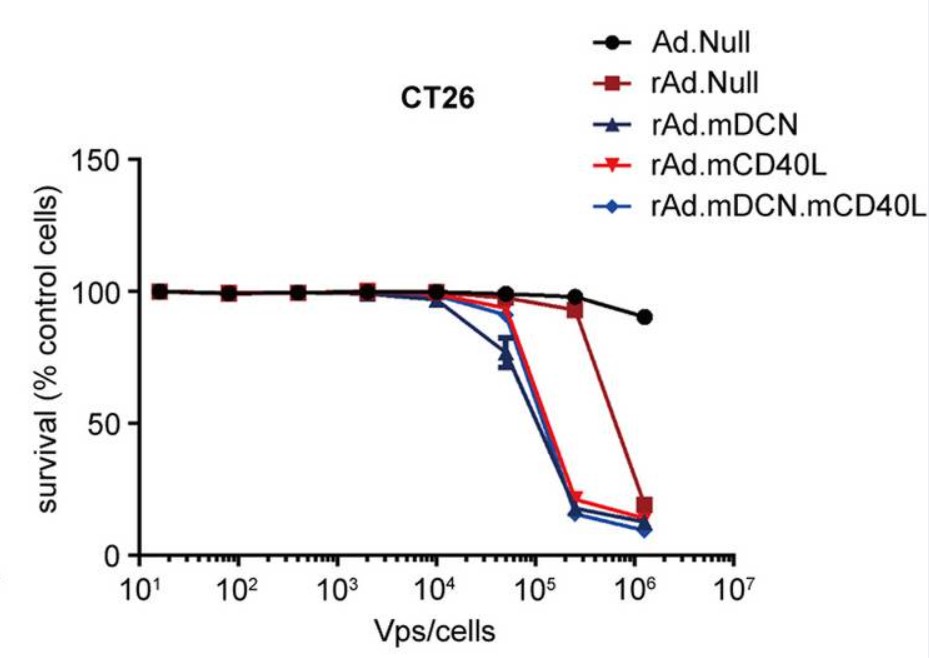
| Fig.2 The genome composition of oncolytic adenovirus loaded with CD40L is constructed.2 | Fig.3 CD40L-loaded oncolytic adenovirus significantly reduced tumor cell survival.2 |
| Flow | Tumor Volume |
|
|
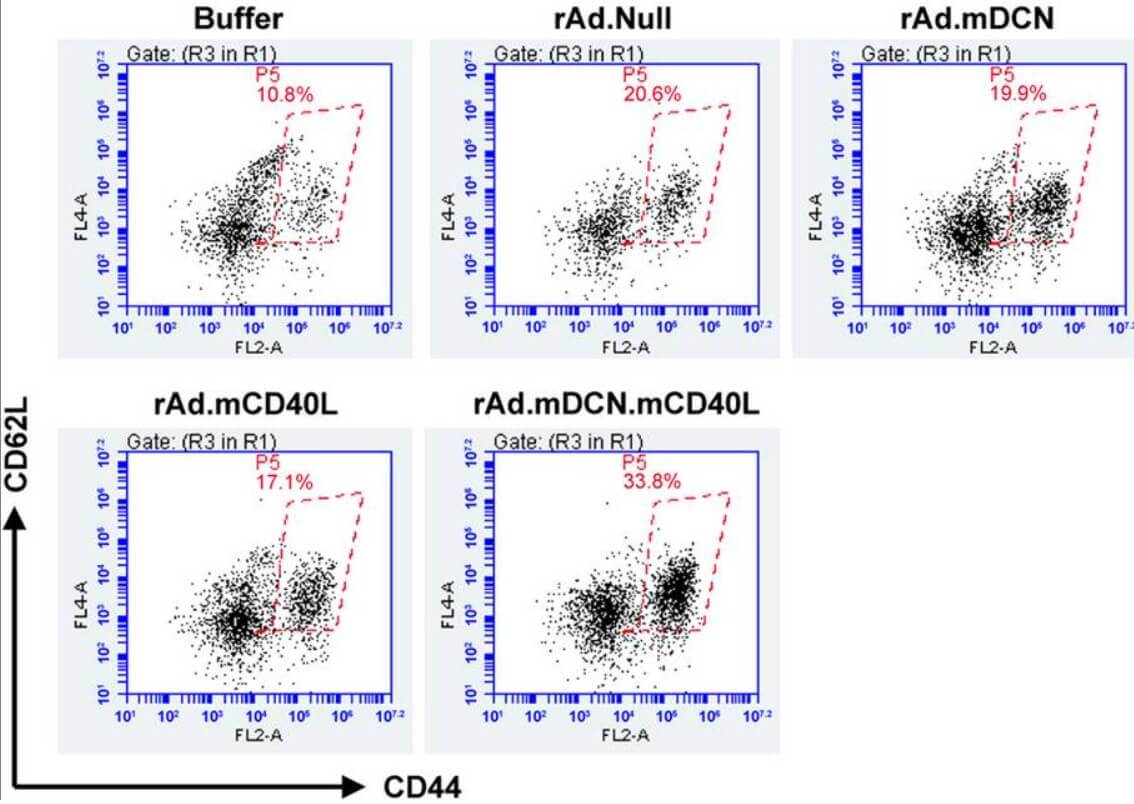
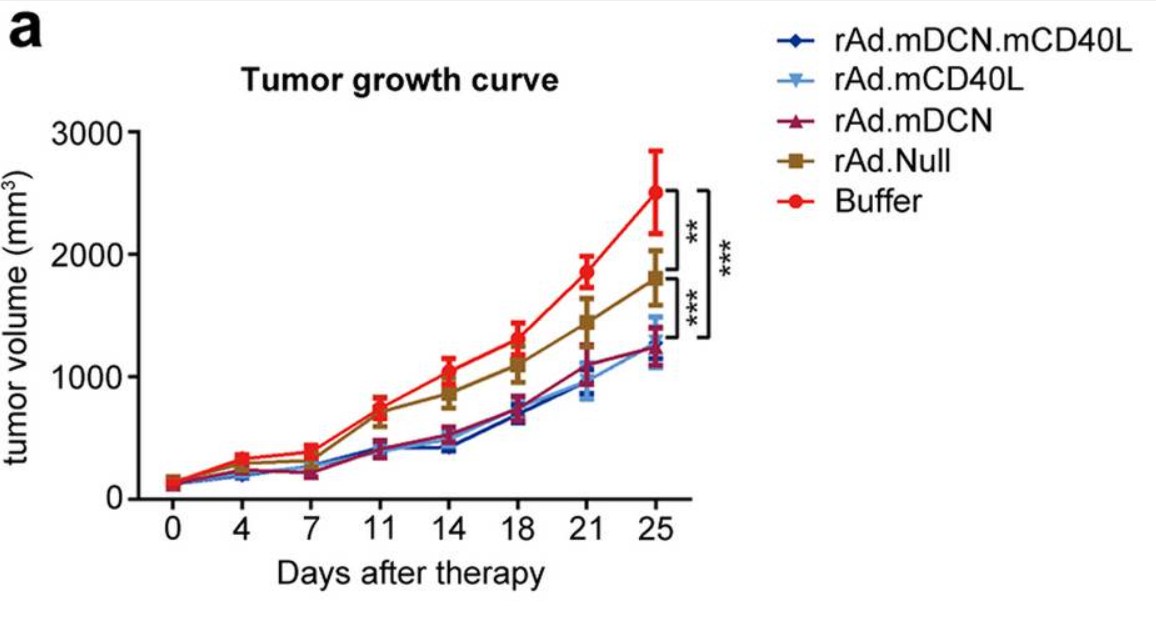
| Fig.4 The effect of CD40L-loaded oncolytic adenovirus on cytotoxic T lymphocytes is detected by flow cytometry.2 | Fig.5 Oncolytic adenovirus loaded with CD40L slowed tumor growth in tumor-bearing mice.2 |
| Body Weight | Tumor Metastasis |
|
|
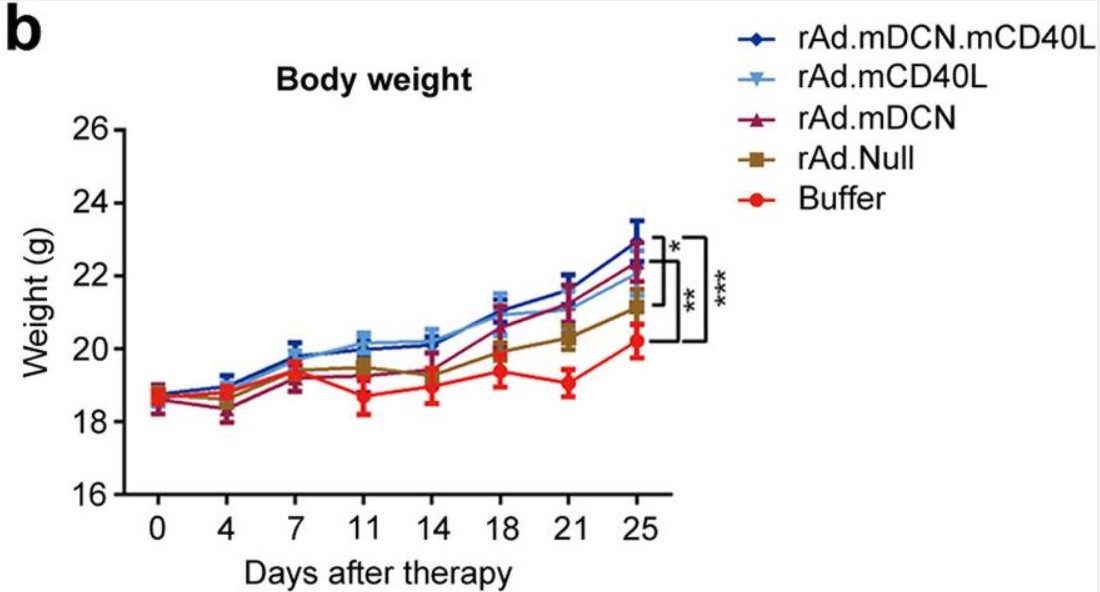
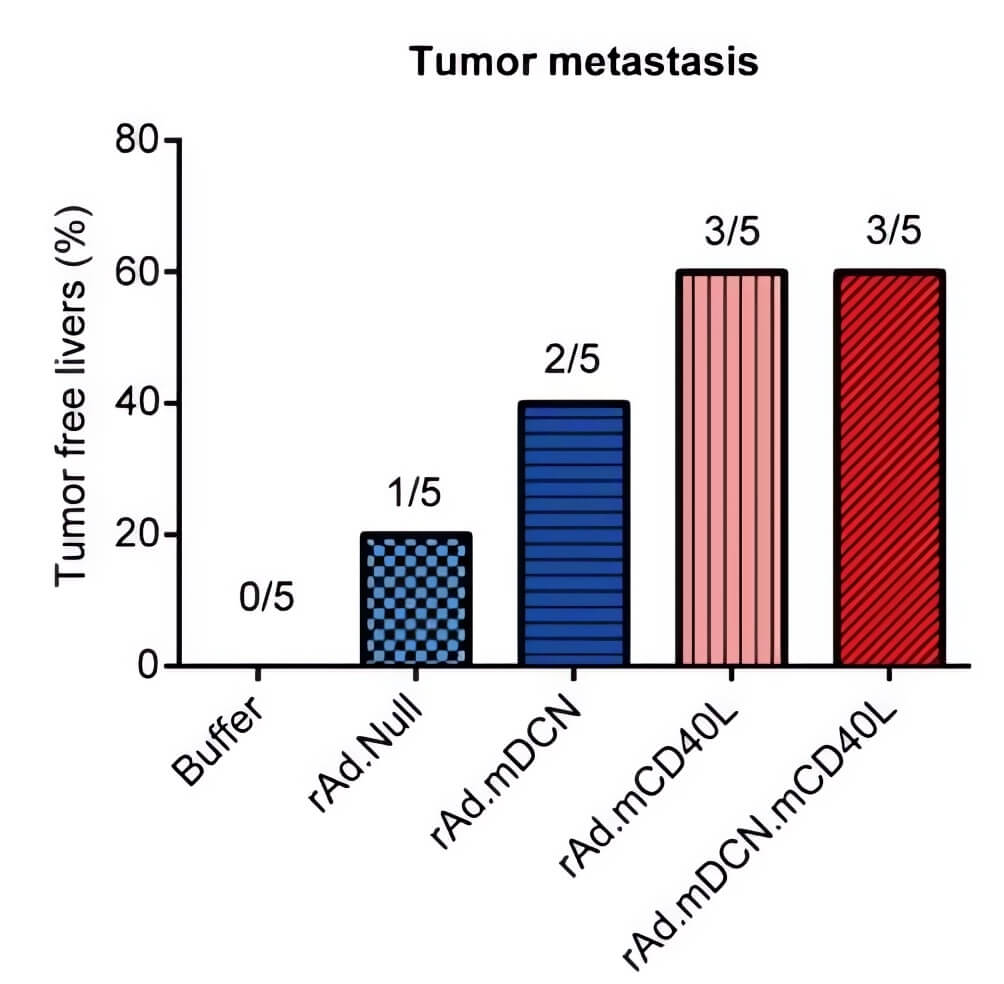
| Fig.6 Oncolytic adenovirus loaded with CD40L can slow down the weight loss of tumor-bearing mice.2 | Fig.7 Tumor metastasis is reduced in tumor-bearing mice treated with oncolytic adenovirus loaded with CD40L.2 |
FAQs
Q1: How does the CD40L-loaded oncolytic adenovirus specifically target cancer cells?
A1: CD40L-loaded oncolytic adenoviruses demonstrate broad potential in solid tumors, especially those requiring immune activation and tumor microenvironment modulation. Preclinical studies show robust efficacy in challenging cancers (e.g., high-grade serous ovarian cancer, colorectal cancer), with translatable mechanisms for diverse indications.
Q2: What types of cancers are most suitable for treatment with CD40L-loaded oncolytic adenoviruses?
A2: CD40L-loaded oncolytic adenoviruses show broad potential in solid tumors, especially those needing immune activation and TME modulation. Preclinical studies prove efficacy in HGSOC, CRC, and other challenging cancers, with widely applicable mechanisms.
Q3: How does the addition of CD40L enhance the therapeutic effect compared to an unmodified oncolytic virus?
A3: The incorporation of CD40L converts oncolytic viruses into potent immunomodulators. Beyond direct tumor lysis, CD40L selectively activates antigen-presenting cells in the tumor microenvironment, driving robust, sustained anti-tumor immunity. This involves enhanced T cell priming/infiltration and a favorable immune landscape shift, significantly improving therapeutic efficacy.
Q4: Can CD40L-loaded oncolytic adenoviruses be used in combination with other existing cancer therapies?
A4: Our CD40L-loaded oncolytic adenovirus platform offers strong synergistic potential with immune checkpoint inhibitors, chemotherapy, and targeted therapies. By reprogramming the tumor microenvironment and stimulating systemic anti-tumor immunity, it enhances the efficacy of complementary treatments. We assist in designing tailored combination strategies to meet your specific therapeutic goals.
Q5: What kind of data or support can I expect from Creative Biolabs during a project involving CD40L-loaded oncolytic adenoviruses?
A5: Creative Biolabs offers end-to-end project support, from design consultation to data delivery. Deliverables include detailed reports on in vitro validation, in vivo efficacy (tumor growth curves, survival data), and immune profiling (flow cytometry, cytokine assays). Our team provides expert data interpretation and strategic recommendations for subsequent steps.
Creative Biolabs is dedicated to advancing cancer immunotherapy with innovative, precisely engineered biotherapeutics. Our CD40L-loaded Oncolytic Adenovirus platform empowers researchers and pharmaceutical companies to develop effective, targeted solid tumor therapies. By merging direct oncolysis with potent immune activation, we provide a comprehensive solution to reshape the tumor microenvironment and drive robust, enduring anti-tumor responses.
Contact Our Team to Discuss Your Project!
Related Sections
| Cytosine Deaminase loaded Oncolytic Adenovirus | Thymidine Kinase loaded Oncolytic Adenovirus |
| Prodrugs loaded Oncolytic Adenovirus | GMCSF loaded Oncolytic Adenovirus |
| hNIS loaded Oncolytic Adenovirus | TNF-α loaded Oncolytic Adenovirus |
| IL-2 loaded Oncolytic Adenovirus | 41BBL loaded Oncolytic Adenovirus |
| PH20 Hyaluronidase loaded Oncolytic Adenovirus | Anti-CTLA4 loaded Oncolytic Adenovirus |
| Anti-PD1 loaded Oncolytic Adenovirus | IL-12 loaded Oncolytic Adenovirus |
| Decorin loaded Oncolytic Adenovirus | OX40L loaded Oncolytic Adenovirus |
| EGFR loaded Oncolytic Adenovirus | FRα loaded Oncolytic Adenovirus |
| FAP loaded Oncolytic Adenovirus | CD44v6 loaded Oncolytic Adenovirus |
References
- Zhang, Shungang, et al. "CD40/CD40L signaling as a promising therapeutic target for the treatment of renal disease." Journal of Clinical Medicine 9.11 (2020): 3653. DOI: 10.3390/jcm9113653. Distributed under Open Access license CC BY 4.0, without modification.
- Rong, Yejing, et al. "Oncolytic adenovirus encoding decorin and CD40 ligand inhibits tumor growth and liver metastasis via immune activation in murine colorectal tumor model." Molecular Biomedicine 5.1 (2024): 39. DOI: 10.1186/s43556-024-00202-1. Distributed under Open Access license CC BY 4.0, the figures were cropped.
