IL-2 loaded Oncolytic Adenovirus Engineering Service
Introduction
Creative Biolabs' IL-2-loaded Oncolytic Adenovirus service accelerates cancer immunotherapy development by leveraging advanced viral engineering and immunomodulation to precisely modulate the tumor microenvironment (TME). Engineered to directly lyse tumor cells and re-educate the TME into a pro-inflammatory, immune-responsive state, this versatile platform overcomes solid tumor challenges, especially immunosuppression, by enhancing infiltration and activation of anti-tumor immune cells. Customized constructs deliver tangible outcomes, including characterized viral vectors, detailed immunological profiling, and preclinical efficacy data to de-risk downstream development.
[Discover How We Can Help - Request a Consultation]
IL-2-loaded Oncolytic Adenovirus
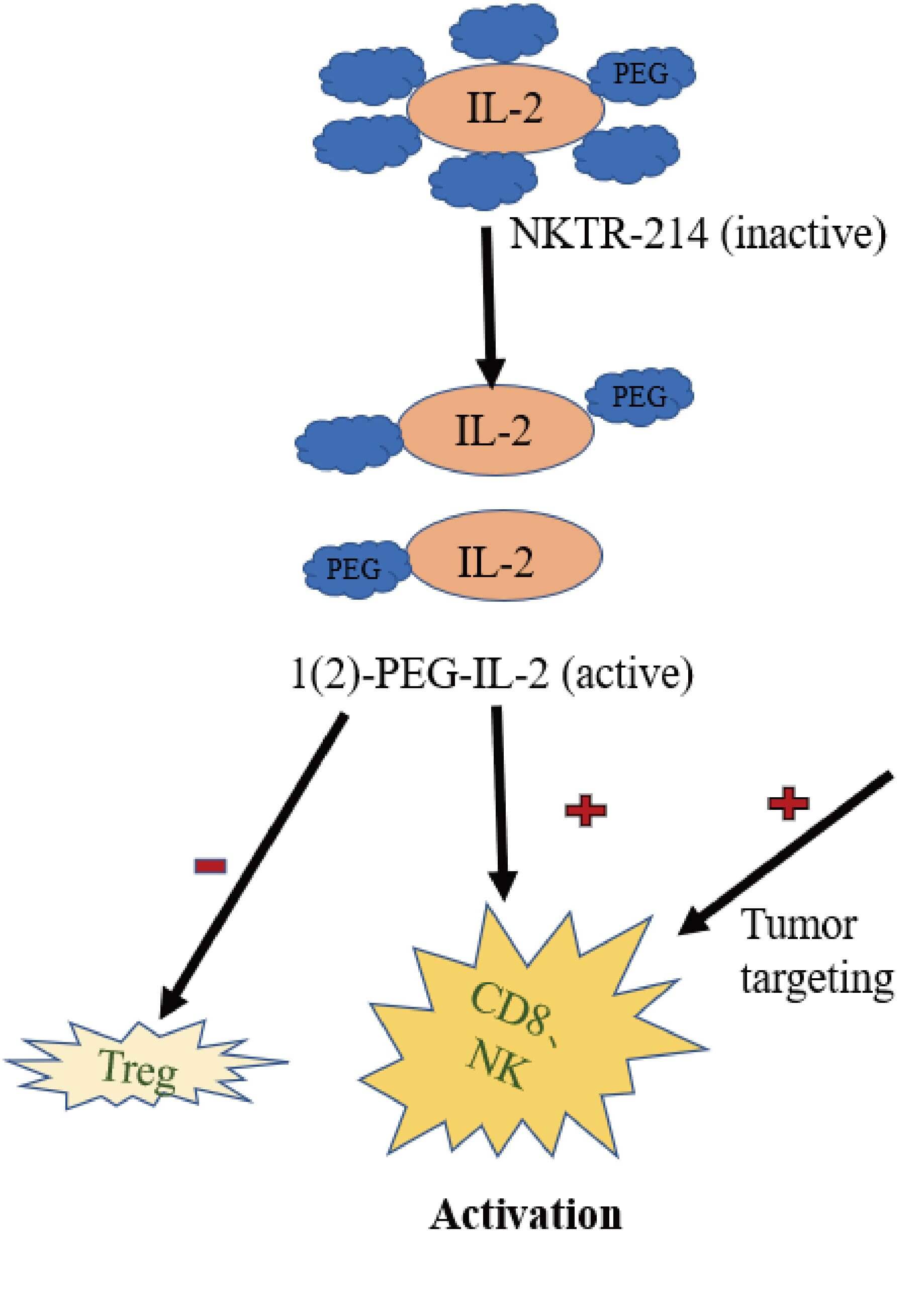 Fig.1 The influence of IL-2 cytokines on immune cells is mainly manifested as inhibiting the function of Treg cells and promoting the functions of NK cells and CD8+ T cells.1
Fig.1 The influence of IL-2 cytokines on immune cells is mainly manifested as inhibiting the function of Treg cells and promoting the functions of NK cells and CD8+ T cells.1
Interleukin-2 (IL-2) is a crucial cytokine renowned for its role in the proliferation, differentiation, and survival of T lymphocytes, particularly cytotoxic T cells (CTLs), which are central to anti-tumor immunity. When delivered directly into the tumor by an oncolytic adenovirus, IL-2 can locally boost immune responses without systemic toxicity often associated with recombinant IL-2 therapies.
Principle
-
Direct Oncolysis
Adenovirus selectively replicates in and lyses tumor cells, releasing TAAs and DAMPs to reduce tumor burden and act as an in situ vaccine. -
Immune Cell Activation
Released DAMPs and viral PAMPs trigger innate immunity, activating pathways like AIM2 inflammasome to promote a pro-inflammatory TME. -
T Cell Proliferation and Recruitment
Locally expressed IL-2 stimulates TIL proliferation/activation (CD4+/CD8+ T cells), enhancing cytotoxicity and sustained tumor infiltration. -
Reprogramming the TME
Combined effects shift the TME from immunosuppressive (MDSCs, Tregs) to immunostimulatory, repressing genes like CD11b/ARG1 and upregulating cytotoxic genes (CD3G, PRF1, GZMK).
Advantages
- Potent Efficacy
IL-2-loaded oncolytic adenoviruses show compelling preclinical results, including significant tumor regression and long-term survival (e.g., 62.5% complete response in monotherapy models).
- Targeted Immune Modulation
Localized IL-2 delivery to tumors avoids systemic toxicities of exogenous IL-2, enabling focused immune stimulation.
- Enhanced T Cell Response
The platform boosts tumor-specific CTL activity, increases IFN-γ release, and recruits critical immune cells to amplify anti-tumor immunity.
- Versatile Platform
Well-characterized adenoviral vectors allow payload customization for various solid tumors, including hard-to-treat types like HCC.
- Combination Potential
TME-reprogramming capabilities make it ideal for synergizing with checkpoint inhibitors or other immunotherapies to enhance anti-tumor effects.
Workflow
| Required Starting Materials | Vector Design and Engineering |
|---|---|
|
Genetically engineer oncolytic adenoviral vectors to integrate interleukin-2 (IL-2) or variant IL-2 (vIL-2), incorporating elements for tumor-selective replication and safety. |
| Viral Production and Purification | In Vitro Validation and Characterization |
| Produce high-titer, high-purity IL-2-loaded OAV batches in state-of-the-art facilities with strict QC for consistency and sterility. | Assess viral replication in tumor/non-tumor cells, quantify IL-2 expression, and evaluate cell lysis and immune pathway activation in cell culture models. |
| In Vivo Efficacy Studies | Immunological Profiling and Mechanistic Elucidation |
| Test therapeutic potential in animal models (syngeneic/PDX) via intratumoral/IV administration, monitoring tumor growth, survival, and immunological responses. | Analyze TME and systemic immunity using flow cytometry (immune cell populations), gene expression (DAMP/PAMP, inflammasome markers), and cytokine profiling (e.g., IFN-γ) to define immune modulation mechanisms. |
| Final Deliverables | Estimated Timeframe |
|
The typical timeframe for this service ranges from 12 to 18 weeks, depending on the complexity of the viral construct, the inclusion of optional preclinical evaluation, and the specific requirements of your project. More intricate designs or extensive in vivo studies may extend the duration. |
[Contact Us for a Personalized Workflow]
What We Can Offer
As a leading provider in oncolytic virotherapy, Creative Biolabs' IL-2-loaded Oncolytic Adenovirus service is meticulously designed to meet the advanced needs of biology experts and drug developers. We offer a robust platform that provides not just a product, but a comprehensive solution for enhancing cancer immunotherapy.
Customized Viral Vector Design and Engineering
Tailored genetic engineering of oncolytic adenoviruses to integrate IL-2 variants or other immunomodulatory payloads for specific research goals and target indications.
High-Quality Viral Production and Purification
State-of-the-art facilities for high-titer, high-purity IL-2-loaded OAV production, meeting strict QC standards for preclinical/clinical use.
Comprehensive In Vitro and In Vivo Efficacy Assessment
Rigorous evaluation of oncolytic activity, IL-2 expression, immune modulation, tumor regression, and survival in preclinical models to de-risk development.
In-depth Immunological Profiling and Mechanistic Insight
Advanced analysis of TME via flow cytometry, gene expression (DAMP/PAMP, inflammasome), and cytokine assays to clarify immune activation mechanisms.
Strategic Consultation and Collaborative Support
Expert guidance from biology specialists for experimental design, data interpretation, and strategic development of IL-2-loaded OAV therapies.
Scalable Solutions for Research to Preclinical Development
Scalable support from early discovery to IND-enabling studies, ensuring project continuity and efficiency.
[Experience the Creative Biolabs Advantage - Get a Quote Today]
Case Study
The utilization of IL-2-loaded oncolytic adenoviruses in preclinical research models and tumor cell lines enhances tumor-specific lytic activity. Investigative results validate its effectiveness for managing solid tumors, highlighting the virus's dual-mechanism strategic approach.
| Construction of Oncolytic Adenovirus Vector | |
|---|---|
|
|
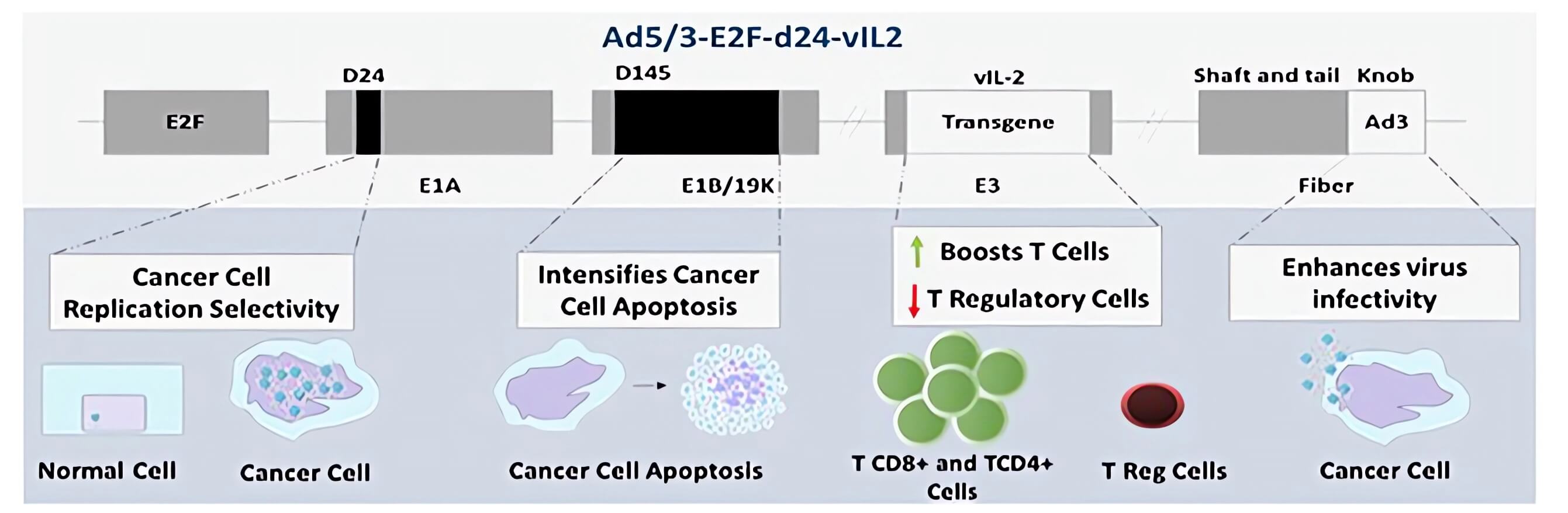
| Fig.2 Schematic representation of the oncolytic adenovirus genome with the addition of variant IL-2.2 | |
| Cell Cytotoxicity | Immune Cell Changes |
|
|
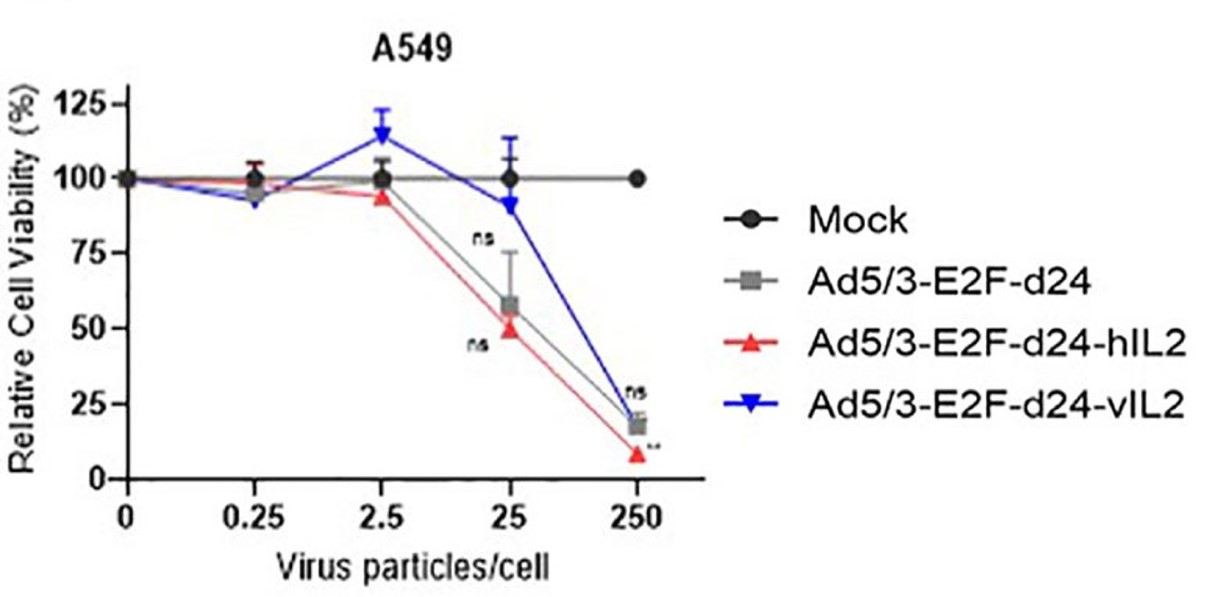
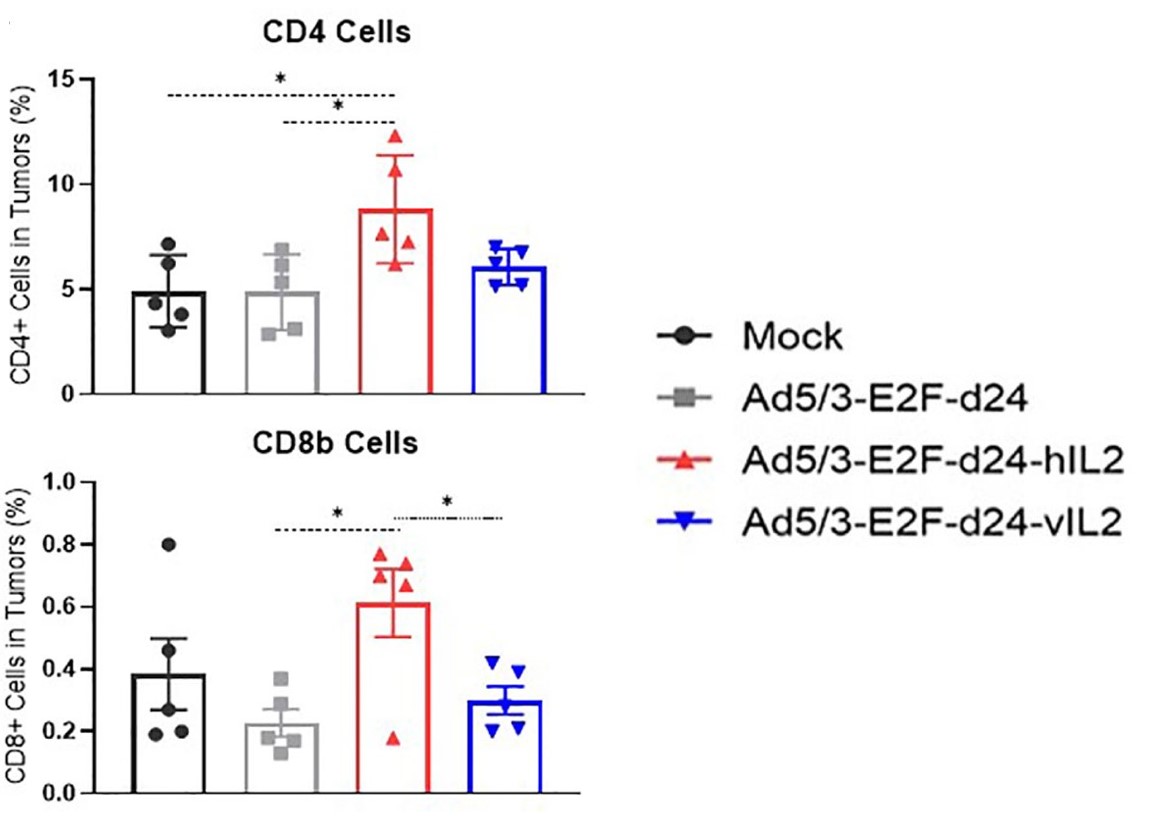
| Fig.3 The effect of IL-2 loaded oncolytic adenovirus and control oncolytic adenovirus on the activity of tumor cells was detected.2 | Fig.4 The changes of CD4+ and CD8+ cells were detected by flow cytometry.2 |
| Tumor Volume | Survival Curve |
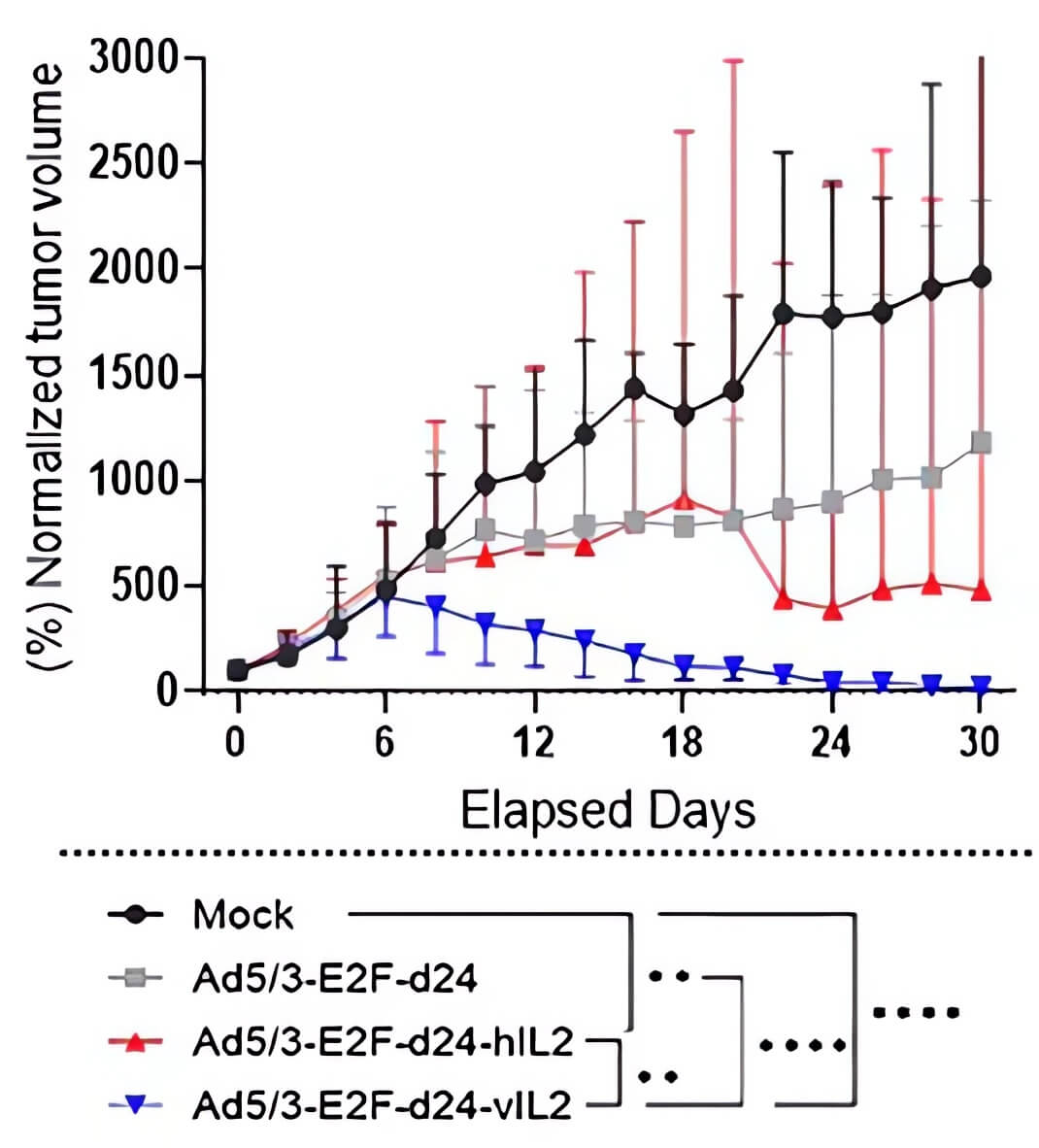
|
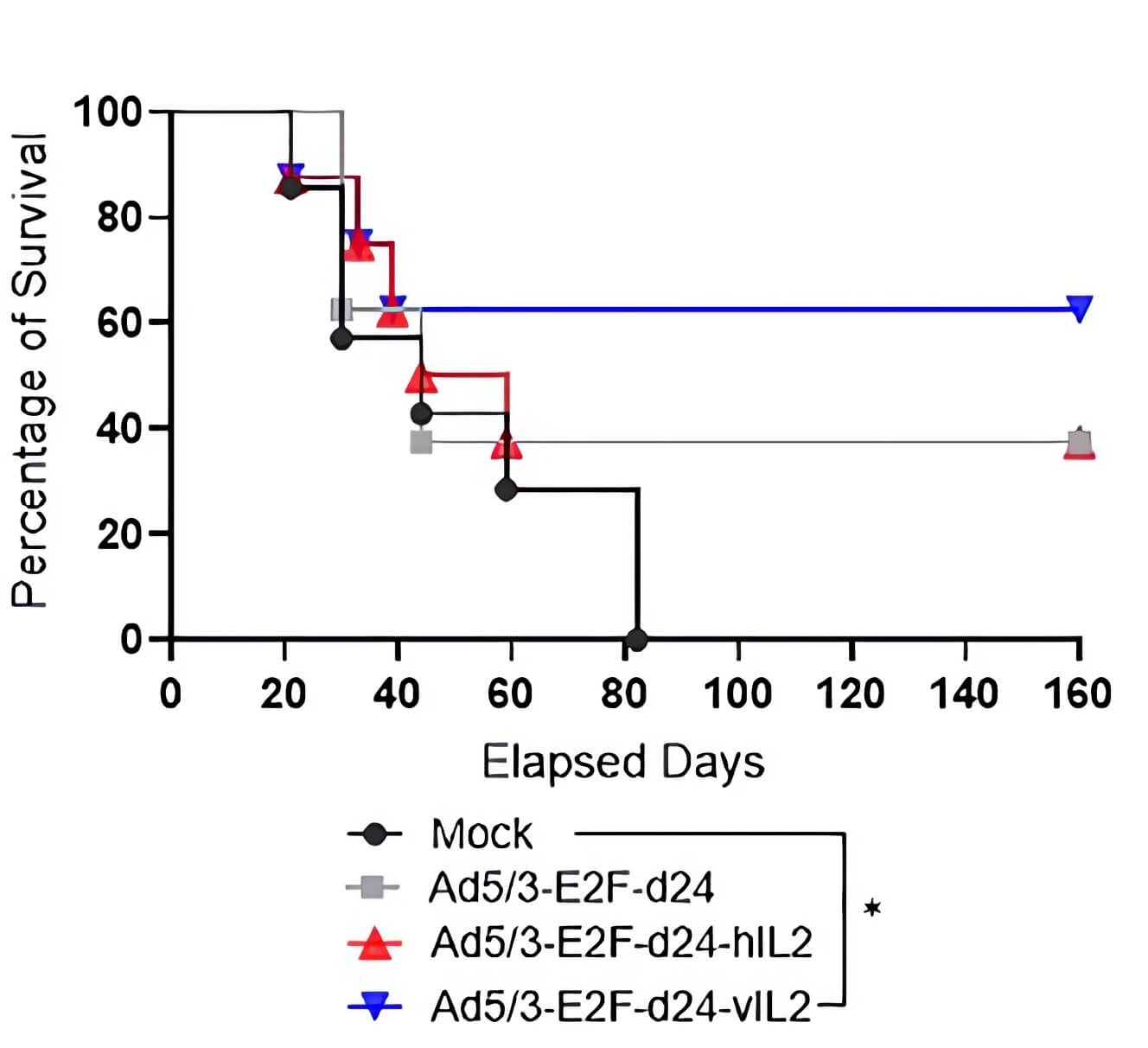
|
| Fig.5 Oncolytic adenovirus loaded with IL-2 to slow tumor growth in tumor-bearing mice.2 | Fig.6 Oncolytic adenovirus loaded with IL-2 prolonged the survival of tumor-bearing mice.2 |
FAQs
How can IL-2-loaded oncolytic adenovirus avoid cytokine storm induced by IL-2 systemic exposure?
Drives IL-2 expression through tumor-specific promoters (such as E2F), and combines with the Ad5/35 chimeric capsid to reduce the infection rate of normal tissues, so that IL-2 is only released at high concentrations in the tumor (systemic concentration<0.1 ng/mL). Preclinical data showed that this strategy reduced the peak serum IL-6 in mice by 92% compared with intravenous IL-2, avoiding systemic inflammatory responses.
What are the advantages of IL-2 OAV over conventional IL-2 adoptive cell therapy?
IL-2 OAV produces a sustained release of IL-2 in tumor situ, avoiding the problem of short IL-2 half-life (<60 min) in adoptive cell therapy. A porcine pancreatic cancer model showed that the infiltration of TILs in the IL-2 OAV group lasted for 21 days, which was 3.5 times longer than that in the IL-2 intravenous infusion group, and the proportion of effector T cells (CD8+ CD107a+) was higher.
Can IL-2-loaded Oncolytic Adenovirus be combined with other cancer treatments?
Absolutely. The mechanism of action, which involves reprogramming the tumor microenvironment, makes our IL-2-loaded oncolytic adenovirus highly suitable for combination therapies. It can potentially synergize with existing immunotherapies, chemotherapy, or radiation, enhancing overall anti-tumor responses.
What kind of data can I expect to receive from your IL-2-loaded Oncolytic Adenovirus service?
You will receive comprehensive data including in vitro viral characterization, detailed in vivo tumor growth and survival curves, and extensive immunological profiling reports from the tumor microenvironment and systemic compartments. This data provides clear insights into the therapeutic efficacy and mechanistic insights.
Creative Biolabs' IL-2-loaded Oncolytic Adenovirus service pioneers' cancer immunotherapy, using scientific expertise and advanced tech to overcome tumor immunosuppression and drive anti-tumor responses. We offer customized solutions to speed up drug discovery. Partner with us to maximize oncolytic virotherapy's potential and revolutionize cancer treatment.
[Contact Our Team for More Information and to Discuss Your Project]
Related Sections
| Cytosine Deaminase loaded Oncolytic Adenovirus | Thymidine Kinase loaded Oncolytic Adenovirus |
| Prodrugs loaded Oncolytic Adenovirus | GMCSF loaded Oncolytic Adenovirus |
| CD40L loaded Oncolytic Adenovirus | hNIS loaded Oncolytic Adenovirus |
| TNF-α loaded Oncolytic Adenovirus | 41BBL loaded Oncolytic Adenovirus |
| PH20 Hyaluronidase loaded Oncolytic Adenovirus | Anti-CTLA4 loaded Oncolytic Adenovirus |
| Anti-PD1 loaded Oncolytic Adenovirus | IL-12 loaded Oncolytic Adenovirus |
| Decorin loaded Oncolytic Adenovirus | OX40L loaded Oncolytic Adenovirus |
| EGFR loaded Oncolytic Adenovirus | FRα loaded Oncolytic Adenovirus |
| FAP loaded Oncolytic Adenovirus | CD44v6 loaded Oncolytic Adenovirus |
References
- Zhou, Yangyihua, et al. "The application of Interleukin-2 family cytokines in tumor immunotherapy research." Frontiers in Immunology 14 (2023): 1090311. DOI: 10.3389/fimmu.2023.1090311. Distributed under Open Access license CC BY 4.0, without modification.
- Quixabeira, Dafne CA, et al. "Oncolytic adenovirus coding for a variant interleukin 2 (vIL-2) cytokine re-programs the tumor microenvironment and confers enhanced tumor control." Frontiers in Immunology 12 (2021): 674400. DOI: 10.3389/fimmu.2021.674400. Distributed under Open Access license CC BY 4.0, the figures were cropped.
