TNFalpha loaded Oncolytic Adenovirus Engineering Service
Introduction
Creative Biolabs' TNFα-loaded Oncolytic Adenovirus service revolutionizes cancer treatment by re-engineering the tumor microenvironment (TME) through advanced oncolytic virotherapy and targeted cytokine delivery. This solution overcomes limitations of conventional immunotherapies, such as low solid tumor efficacy and systemic toxicities, by stimulating robust anti-tumor immunity. Leveraging the multi-modal actions of TNF-α, the service enhances immune responses within the tumor, delivering safer and more effective therapeutic outcomes tailored to address challenges in solid tumor treatment.
TNFα-loaded Oncolytic Adenovirus
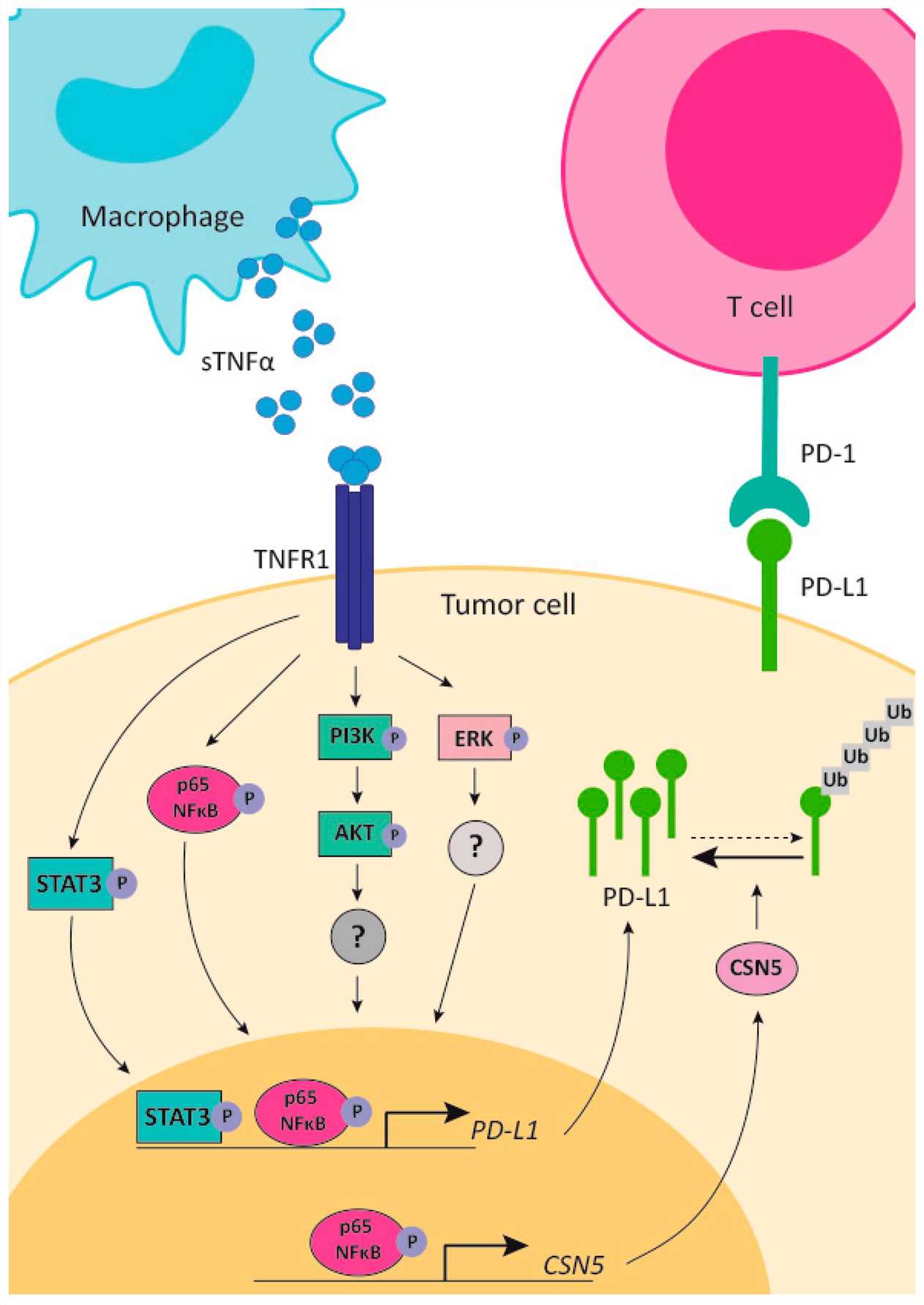
Tumor Necrosis Factor alpha (TNF-α) is a pleiotropic cytokine with a complex yet critical role in immunity and cancer. Historically recognized for its potent pro-inflammatory and direct cytotoxic effects on tumor cells, its systemic administration has been hampered by severe dose-limiting toxicities. The advent of oncolytic adenoviruses provides an elegant solution to deliver TNF-α precisely to the TME, leveraging its therapeutic potential while mitigating systemic side effects.
Fig.1 Schematic representation of the mechanism of action of TNF-α and its related signaling pathways.1
Principle
-
Tumor-Selective Replication
Engineered with E2F promoter and E1A deletions, the OAV preferentially infects and replicates in rapidly dividing cancer cells, sparing healthy tissues. -
Direct Oncolysis
Viral replication in tumor cells triggers immunogenic cell death, releasing viral particles, tumor antigens (TAAs), and danger signals (DAMPs) to activate anti-tumor immunity. -
Localized TNF-α Expression
OAV drives high-level hTNF-α expression in infected tumor cells, enabling targeted cytokine action with a higher therapeutic index than systemic delivery. -
Immune Cell Recruitment and Activation
Local TNF-α attracts and activates CD8+ T cells, CD4+ T cells, and NK cells, inducing anti-tumor inflammation and amplifying immune responses via cytokine/chemokine release. -
Direct Anti-Tumor Effects
High local TNF-α concentrations directly induce necrosis and apoptosis in sensitive tumor cells, providing an additional cytotoxic mechanism.
Advantages
- Enhanced Potency
Oncolysis combined with targeted TNF-α delivery creates synergistic anti-tumor effects, outperforming single-modality treatments.
- Tumor-Specific Targeting
Oncolytic adenoviruses ensure TNF-α activity is localized to tumors, reducing systemic exposure and toxicities.
- Immunogenic Cell Death
Viral oncolysis and TNF-α induce immunogenic cell death, releasing TAAs to trigger systemic anti-tumor immunity and memory responses.
- Versatile Combination Partner
TNF-α-loaded OAVs pair effectively with chemotherapy, radiotherapy, and immune checkpoint inhibitors for next-generation combination therapies.
- Reversal of Immunosuppression
Localized OAV delivery of TNF-α overcomes TME immunosuppression by activating immune cells, while advanced vector design mitigates pro-tumorigenic effects associated with systemic TNF-α signaling.
Workflow
| Required Starting Materials | Project Consultation & Design |
|---|---|
|
In-depth consultation to understand project goals, technical specs, and regulatory needs. Collaborative design of OAV constructs, including promoter selection, hTNF-α transgene insertion, and capsid modifications (e.g., Ad5/3 chimeric capsid). |
| Gene Synthesis & Vector Construction | Virus Production & Purification |
| Synthesis of hTNF-α transgene and integration into OAV backbone via advanced cloning (e.g., BAC-recombineering). | Large-scale production in qualified cell lines, followed by cesium chloride gradient centrifugation for purification. |
| In Vitro Characterization & Validation | In Vivo Efficacy & Safety Evaluation |
| Assessment of viral replication, oncolytic activity in human/hamster cancer cell lines, and hTNF-α expression/bioactivity via cytometric bead arrays and indicator assays. Evaluation of synergy with immune cells. | Testing in immunocompetent (e.g., Syrian hamsters) or immunocompromised (e.g., SCID mice) models. Monitors tumor growth inhibition, survival, systemic cytokine levels, organ histology, and TME immune profiling. |
| Final Deliverables | Estimated Timeframe |
|
The typical timeframe for this service ranges from 12 to 18 weeks, depending on the complexity of the viral construct, the inclusion of optional preclinical evaluation, and the specific requirements of your project. More intricate designs or extensive in vivo studies may extend the duration. |
[Contact Us for a Personalized Workflow]
What we can offer
Tailored OAV Construct Design
Expert collaboration to customize TNFα-loaded OAV constructs, optimizing promoter strength, transgene integration, and capsid serotypes for specific tumor models and therapeutic goals.
High-Yield Virus Production & Purification
State-of-the-art facilities ensure large-scale production of high-titer, high-purity OAVs with minimal batch variability for consistent results.
Comprehensive Functional Validation
In vitro/in vivo assessment of viral oncolytic activity, localized TNF-α expression/bioactivity, and tumor microenvironment immune modulation.
Strategic Immune Enhancement Expertise
Design of OAVs to enhance recruitment/activity of CD8+ T cells and NK cells, transforming immunosuppressive tumor microenvironments.
Accelerated Preclinical Development
Proven workflow and expertise to expedite preclinical timelines, providing critical data for clinical translation.
Guidance for Combination Therapies
Specialized support for integrating TNFα-loaded OAVs with chemotherapy, adoptive cell therapies, and immune checkpoint inhibitors to unlock synergistic effects.
Robust Quality Control & Documentation
Stringent QC measures and comprehensive documentation to support regulatory submissions and research integrity.
Case Study
The application of TNFα-loaded oncolytic adenoviruses in preclinical animal models and cancer cell lines elevates tumor-selective lytic capacity. Investigations confirm its efficacy for solid tumors, underscoring the virus's two-pronged strategic approach.
| Cell Cytotoxicity | Immune cell changes |
|---|---|
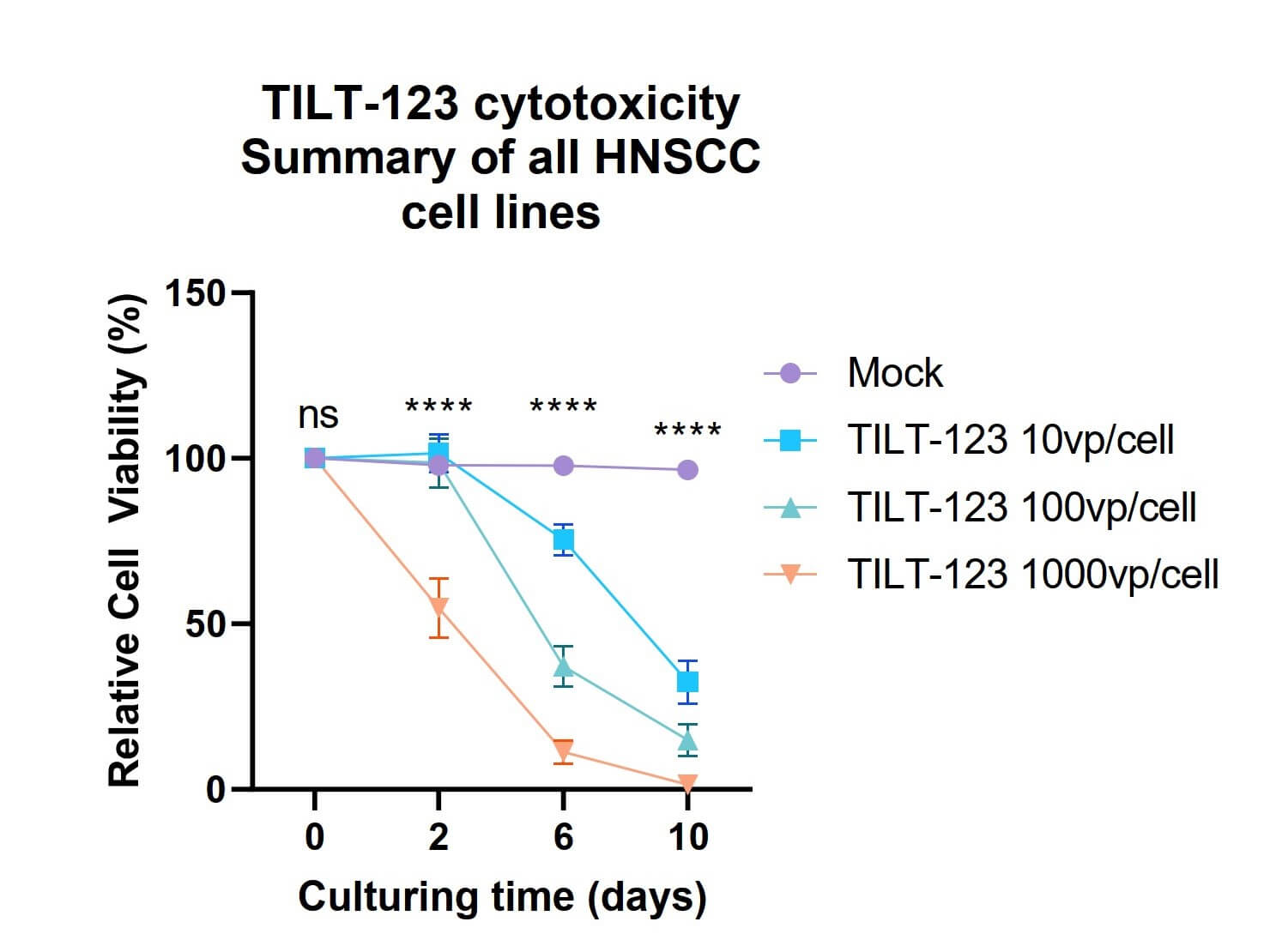
|
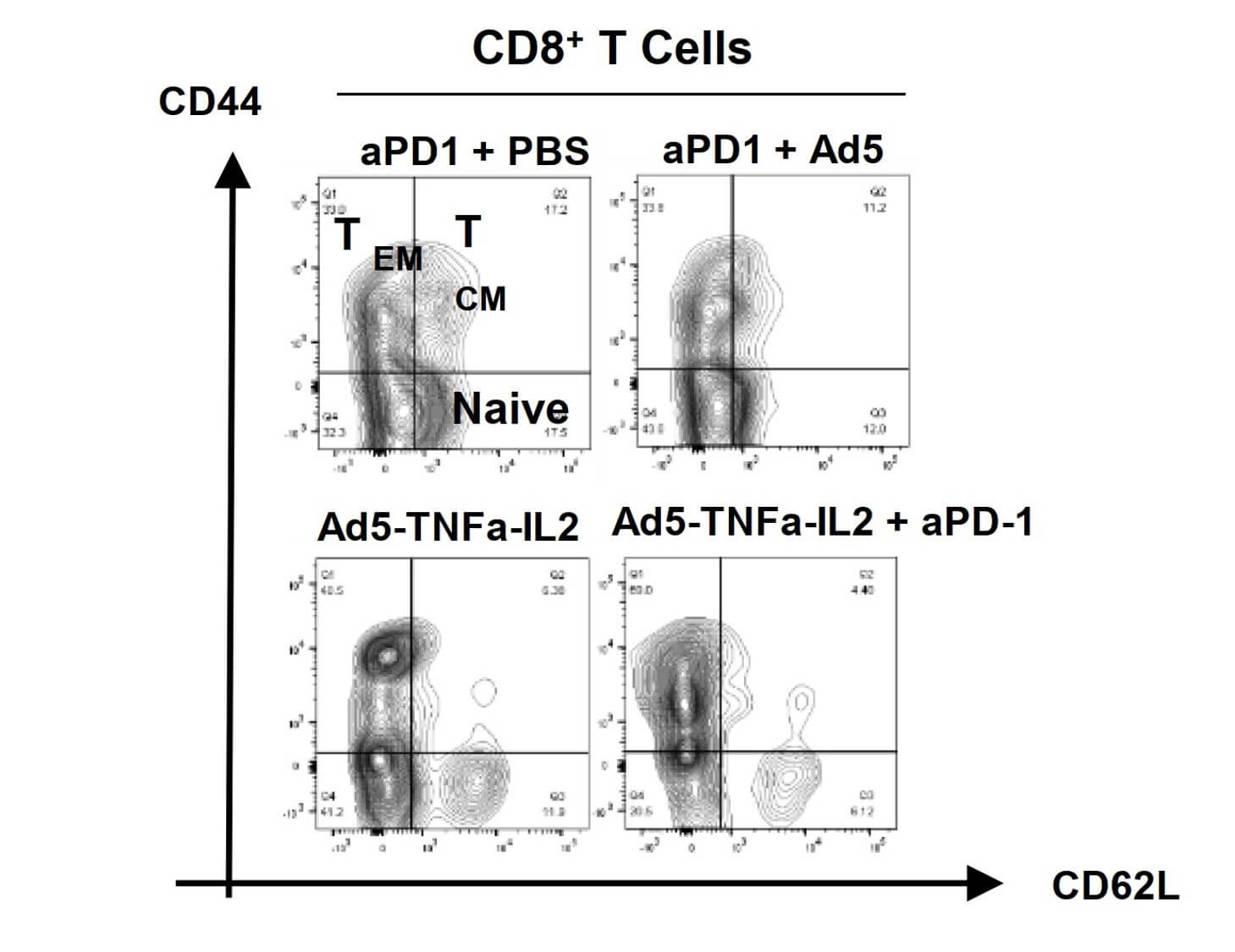
|
| Fig.2 The effect of different concentrations of oncolytic adenovirus loaded with TNF-α on the activity of tumor cells was detected.2 | Fig.3 The changes of CD8+ cells were detected by flow cytometry.2 |
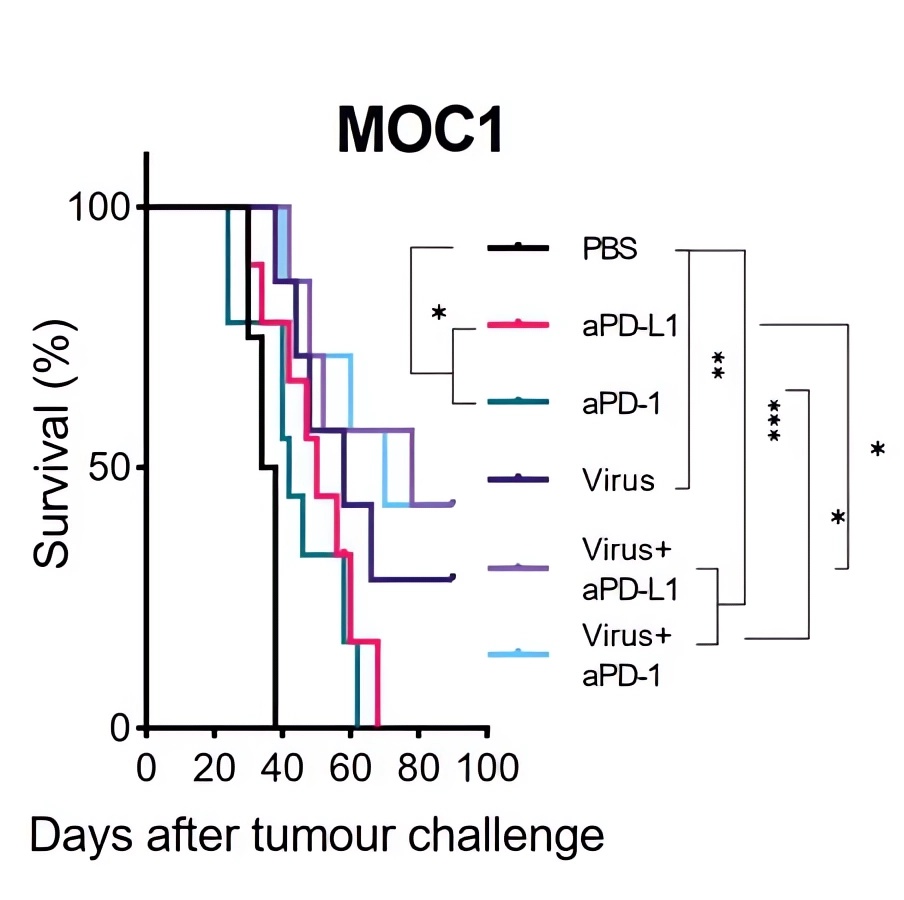
| Tumor Volume | Survival Curve |
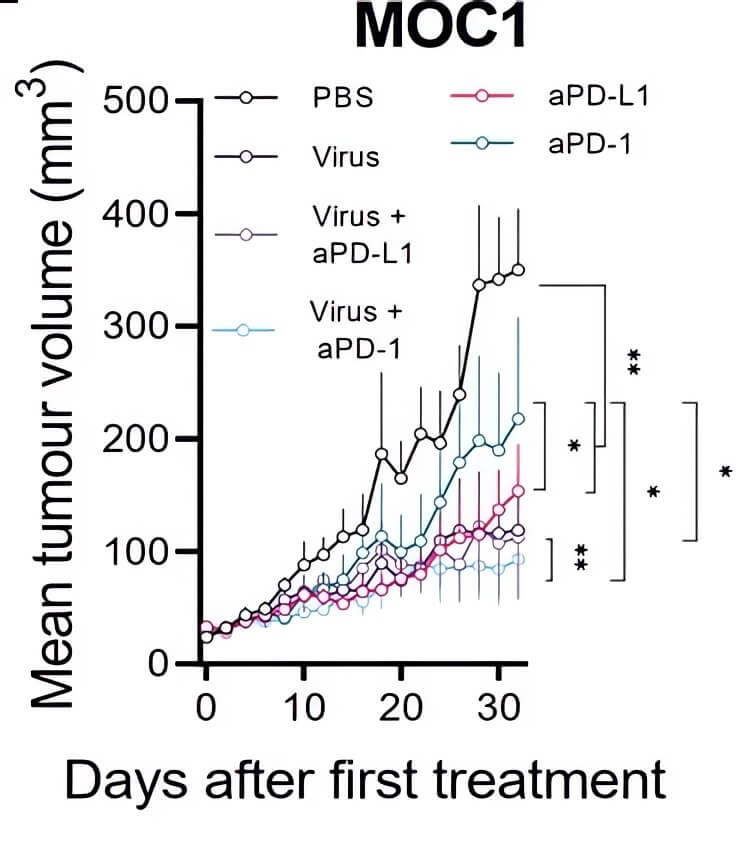
|
|
| Fig.4 Oncolytic adenovirus loaded with TNF-α synergized with immune checkpoint inhibitors to slow tumor growth in tumor-bearing mice.2 | Fig.5 Oncolytic adenovirus loaded with TNF-α combined with immune checkpoint inhibitors prolonged the survival of tumor-bearing mice.2 |
FAQs
How does Creative Biolabs' TNF-α-loaded Oncolytic Adenovirus differ from traditional systemic TNF-α therapies, especially regarding safety?
Our TNF-α-loaded Oncolytic Adenovirus is engineered for tumor-selective replication, enabling TNF-α production directly in the tumor microenvironment. This localized delivery avoids the severe, dose-limiting toxicities of traditional systemic TNF-α administration by minimizing off-target effects. It ensures potent anti-tumor activity with minimal systemic exposure and improved safety.
What types of solid tumors can Creative Biolabs' TNF-α-loaded Oncolytic Adenovirus potentially target?
Our TNF-α-loaded Oncolytic Adenovirus has shown promising efficacy in preclinical solid tumor models, including pancreatic, ovarian, lung, colorectal cancers, and melanoma. Its oncolytic replication and TNF-α-mediated immune stimulation apply to various tumor types, especially those with immunosuppressive microenvironments.
Can this therapy be combined with other existing cancer treatments like chemotherapy or immune checkpoint inhibitors?
Our TNF-α-loaded Oncolytic Adenovirus offers strong synergistic potential with standard therapies. Preclinical data shows it enhances efficacy of chemotherapies and immune checkpoint blockades by remodeling the TME and boosting anti-tumor immunity.
How does Creative Biolabs ensure the optimal balance of TNF-α activity, considering its complex role in the tumor microenvironment?
Our approach focuses on highly controlled, localized TNF-α delivery. Engineered adenoviruses express TNF-α primarily in infected tumor cells, achieving high local concentrations for immune activation and direct tumor killing, while keeping systemic levels undetectable to avoid toxicity. Our extensive preclinical validation and understanding of TNF signaling (TNFR1/TNFR2) enable vector design that optimizes TNF-α's immunostimulatory effects within the oncolytic platform, with precise control as a technological cornerstone.
At Creative Biolabs, we advance cancer immunotherapy with innovative, precisely engineered solutions. Our TNFα-loaded Oncolytic Adenovirus service transforms solid tumor targeting by combining oncolysis with targeted cytokine delivery, enhancing anti-tumor immunity for safer, more effective cancer therapy.
[Contact Our Team for More Information and to Discuss Your Project]
Related Sections
| Cytosine Deaminase loaded Oncolytic Adenovirus | Thymidine Kinase loaded Oncolytic Adenovirus |
| Prodrugs loaded Oncolytic Adenovirus | GMCSF loaded Oncolytic Adenovirus |
| CD40L loaded Oncolytic Adenovirus | hNIS loaded Oncolytic Adenovirus |
| IL-2 loaded Oncolytic Adenovirus | 41BBL loaded Oncolytic Adenovirus |
| PH20 Hyaluronidase loaded Oncolytic Adenovirus | Anti-CTLA4 loaded Oncolytic Adenovirus |
| Anti-PD1 loaded Oncolytic Adenovirus | IL-12 loaded Oncolytic Adenovirus |
| Decorin loaded Oncolytic Adenovirus | OX40L loaded Oncolytic Adenovirus |
| EGFR loaded Oncolytic Adenovirus | FRα loaded Oncolytic Adenovirus |
| FAP loaded Oncolytic Adenovirus | CD44v6 loaded Oncolytic Adenovirus |
References
- Mercogliano, María Florencia, et al. "Harnessing tumor necrosis factor alpha to achieve effective cancer immunotherapy." Cancers 13.3 (2021): 564. DOI: 10.3390/cancers13030564. Distributed under Open Access license CC BY 4.0, without modification.
- Clubb, James HA, et al. "Adenovirus encoding tumor necrosis factor alpha and interleukin 2 induces a tertiary lymphoid structure signature in immune checkpoint inhibitor refractory head and neck cancer." Frontiers in immunology 13 (2022): 794251. DOI: 10.3389/fimmu.2022.794251. Distributed under Open Access license CC BY 4.0, the figures were cropped.
