Thymidine Kinase loaded Oncolytic Adenovirus Engineering Service
Introduction
Creative Biolabs' OncoVirapy™ Platform, featuring Thymidine Kinase-loaded Oncolytic Adenovirus, addresses challenges like limited efficacy, systemic toxicity, drug resistance, and metastatic disease. The precision-engineered platform enables targeted tumor destruction, induces systemic anti-tumor immunity, and reduces systemic toxicity compared to conventional therapies. It supports real-time therapy monitoring, synergistic combinations with other modalities, and is tailored to unique R&D goals, providing a clinically viable path for novel cancer therapeutics.
[Discover How We Can Help - Request a Consultation]
Thymidine Kinase-loaded Oncolytic Adenovirus
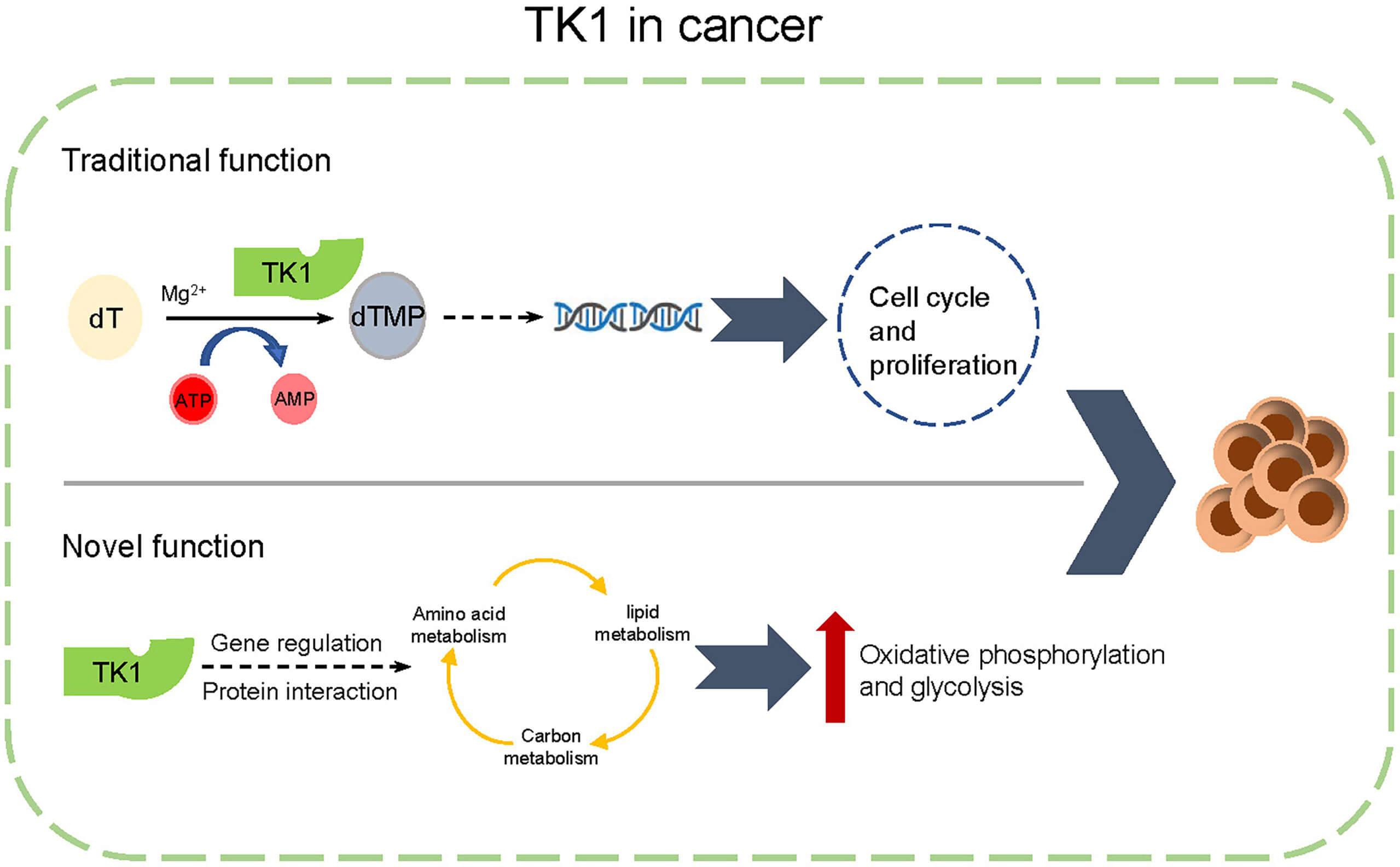 Fig.1 Mechanisms of action of TK-related proteins in tumor cells.1
Fig.1 Mechanisms of action of TK-related proteins in tumor cells.1
The mechanism of action of thymidine kinase (TK) in cancer therapy is mainly based on its enzymatic activation function of prodrugs: specifically, viral thymidine kinase can catalyze the phosphorylation of non-toxic prodrug ganciclovir (GCV) to gradually be converted to the triphosphate form (GCV-TP). This toxic metabolite competitively inhibits DNA polymerase and is incorporated into the DNA strand to terminate synthesis, leading to cancer cell death.
Advantages
- Localized Cytotoxicity: By activating a prodrug only within cancer cells expressing the suicide gene, systemic toxicity is minimized compared to traditional chemotherapy.
- Amplified Therapeutic Effect: The bystander effect ensures that a significant portion of the tumor is destroyed, even if not all cells are directly infected by the virus.
- Broad Applicability: These systems can target both dividing and non-diving cancer cells, which is particularly advantageous for slow-growing tumors where conventional therapies may be less effective.
- Combinatorial Potential: Both TK and CD systems can be combined with other therapeutic payloads (e.g., immunomodulatory genes like sPD1-Ig or cytokines) to create multi-pronged attack strategies, leading to synergistic anti-tumor effects and potent immune activation.
- Clinical Translation: These systems have been extensively studied in preclinical models and have progressed into clinical trials, demonstrating safety and preliminary efficacy.
Workflow
| Required Starting Materials | Project Consultation & Design |
|---|---|
|
In-depth consultation is conducted to understand the project objectives, and OncoVirapy™ Platform is then used to design the best oncolytic adenovirus skeleton. We strategically insert therapeutic genes to achieve therapeutic goals. |
| Vector Engineering & Construction | Virus Production & Purification |
| Employ precision molecular biology and cloning techniques to engineer and construct the recombinant adenoviral vector. Ensure accurate insertion of all therapeutic genes into the viral genome, prioritizing genetic stability and optimal expression. | Produce the engineered oncolytic adenovirus at scale in specialized high-capacity cell lines. Then purify through rigorous multi-stage processes to achieve high titers, purity, and minimal replication-competent adenovirus (RCA) contamination. |
| Preclinical Evaluation (In Vitro and In Vivo) | Data Analysis & Reporting |
| Conduct comprehensive testing in cancer cell lines to assess viral replication, gene expression, and oncolytic efficacy. Perform in vivo studies in animal models to evaluate tumor selectivity, bystander effect, immune activation, and safety. Use PET imaging to track viral activity in real-time. | Analyze all experimental data by bioinformaticians and scientists, then generate comprehensive reports with graphs and statistics. Collaborate with your team to review results, interpret implications, and recommend next steps for development or clinical translation. |
| Final Deliverables | Estimated Timeframe |
|
The typical timeframe for this service ranges from 10 to 15 weeks, depending on the complexity of the viral construct, the inclusion of optional preclinical evaluation, and the specific requirements of your project. More intricate designs or extensive in vivo studies may extend the duration. |
[Contact us to get more details]
What we can offer
- Customized Vector Engineering: Design and development of precision E1E4-deleted adenoviral vectors tailored to specific tumor types and therapeutic payloads.
- Multi-Modal Therapeutic Arming: Integration of suicide genes for localized cytotoxicity, alongside immunomodulatory genes to unleash potent systemic anti-tumor immunity.
- High-Purity, Clinical-Grade Production: Scalable manufacturing of high-titer oncolytic adenovirus vectors with stringent quality control to minimize replication-competent adenovirus (RCA) contamination.
- Comprehensive Preclinical Evaluation: Robust in vitro and in vivo studies, including sophisticated molecular imaging (PET) for real-time tracking of viral efficacy and biodistribution.
- Induction of Durable Anti-Metastatic Immunity: Solutions specifically engineered to stimulate long-lasting immune memory, critical for preventing recurrence and addressing disseminated disease.
- Expert Consultation & Project Guidance: Leverage over two decades of specialized biology and gene therapy expertise from Creative Biolabs dedicated team to navigate your project from concept to preclinical success.
[Experience the Creative Biolabs Advantage - Get a Quote Today]
Case Study
The utilization of Thymidine Kinase-loaded oncolytic adenoviruses in preclinical models and tumor cell lines augments tumor-selective cytotoxicity. Investigations validate its promise for solid neoplasms, underscoring the virus's dual-action mechanism.
| Oncolytic Virus Construction | ID50 |
|---|---|
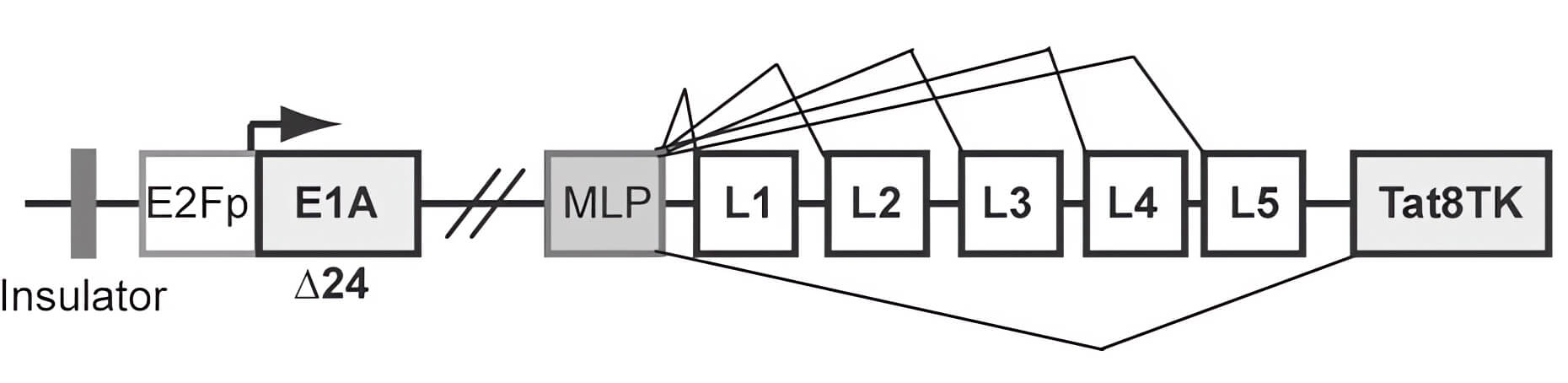
|
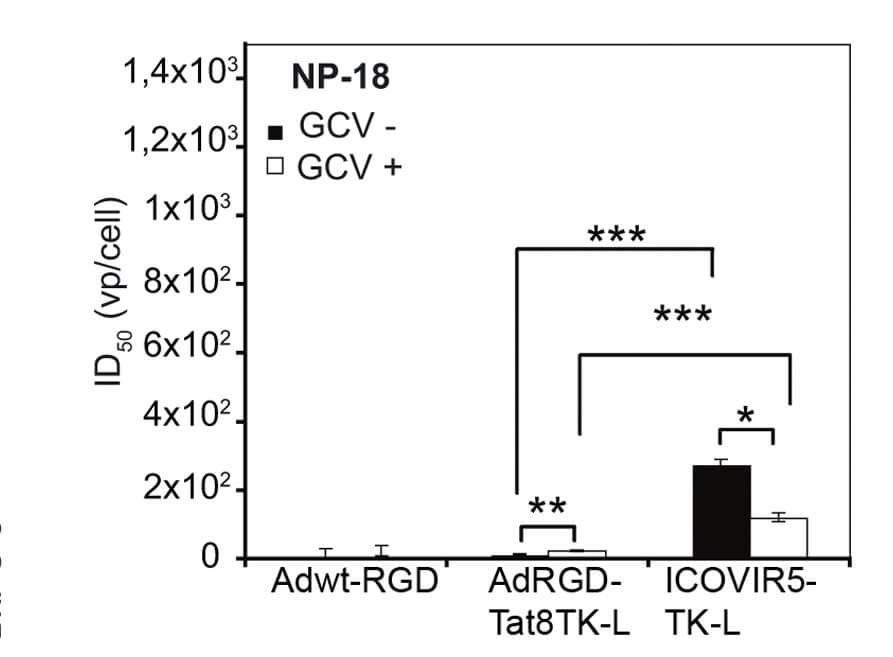
|
| Fig.2 Schematic representation of an oncolytic adenovirus genome loaded with the TK gene.2 | Fig.3 The corresponding ID50 values of pancreatic cancer cells treated with different oncolytic adenoviruses were determined.2 |
| Replication Capacity | Cell Viability |
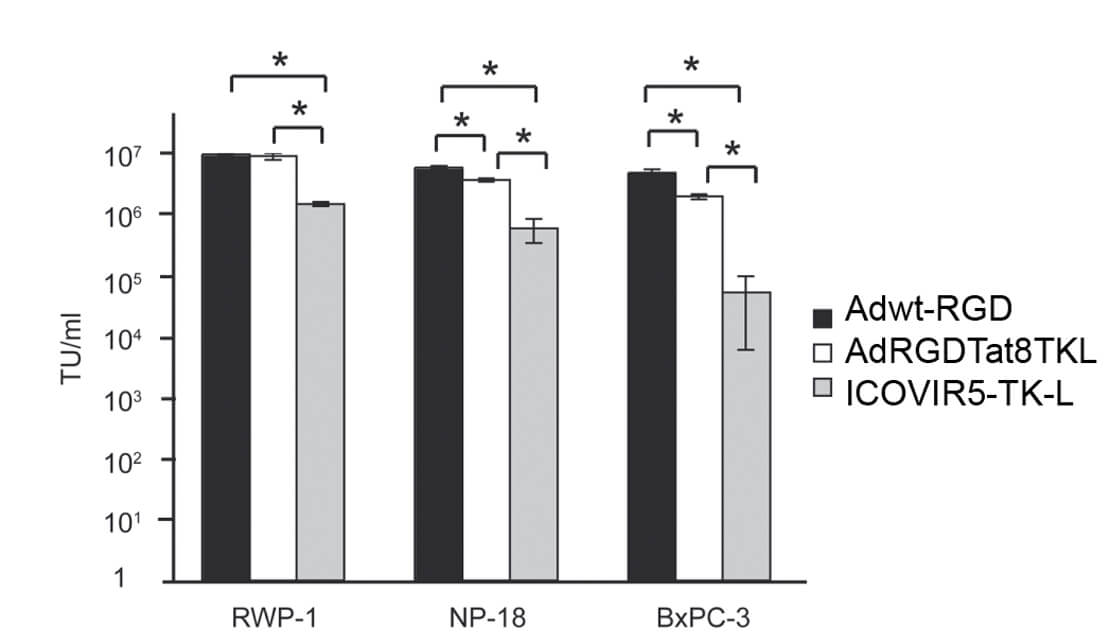
|
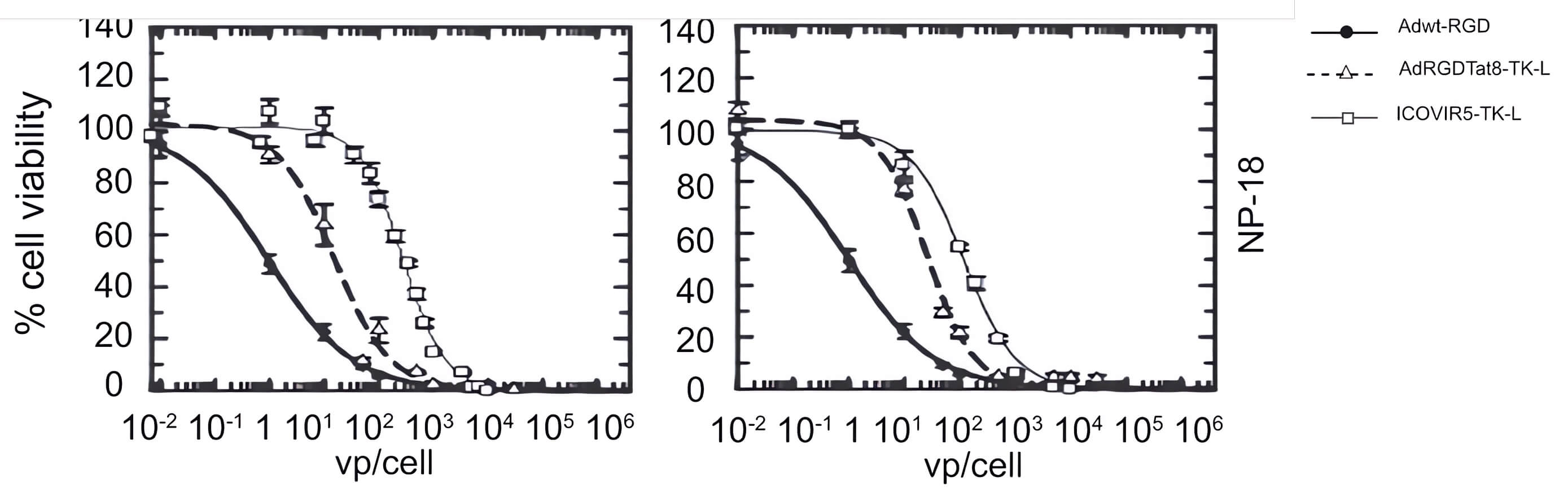
|
| Fig.4 Replication capacity of TK-loaded oncolytic adenovirus in tumor cells.2 | Fig.5 Effect of TK-loaded oncolytic adenovirus on the activity of pancreatic cancer cells.2 |
| Survival Curve | Tumor Volume |
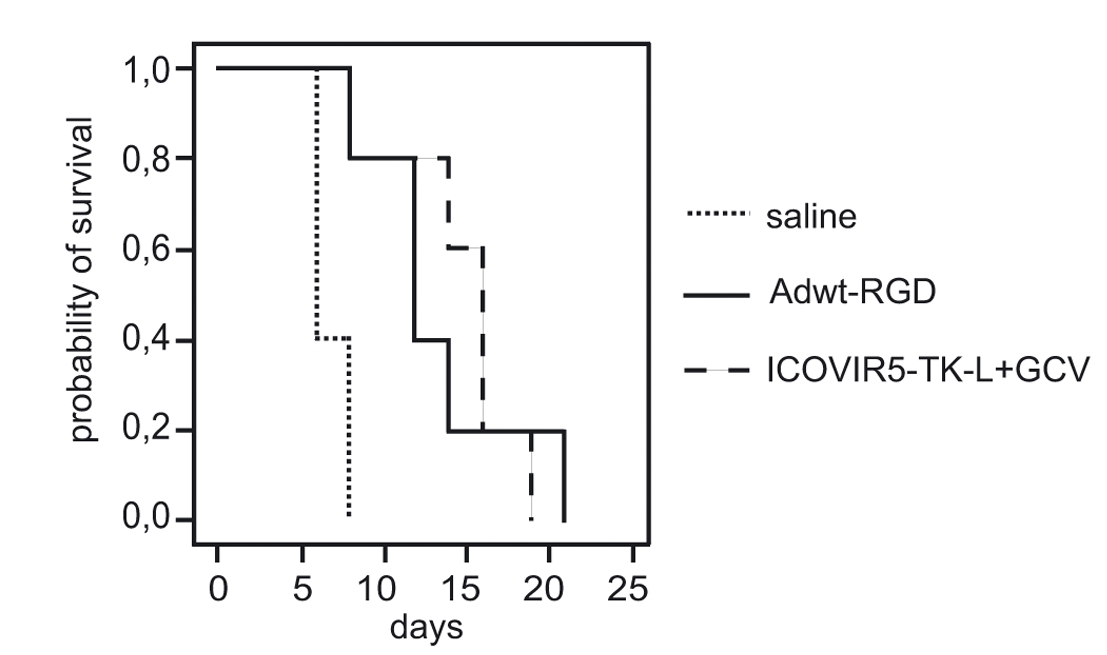
|
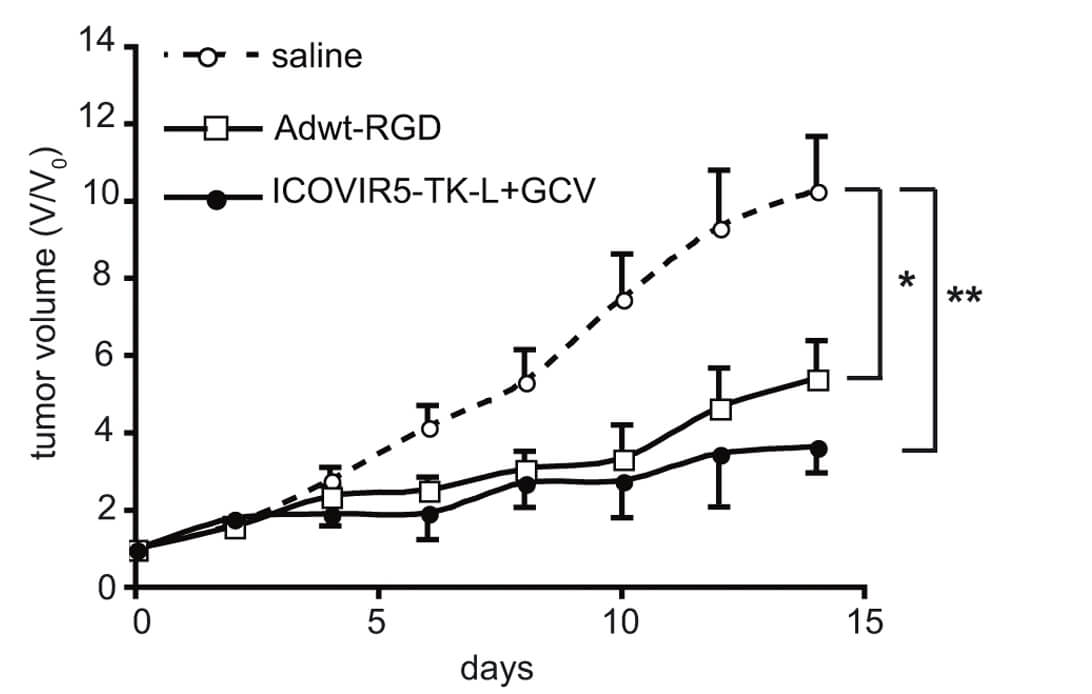
|
| Fig.6 The oncolytic adenovirus loaded with TK could prolong the survival time of tumor-bearing mice.2 | Fig.6 Tk-loaded oncolytic adenovirus alone or in combination with ganciclovir slowed the rate of tumor growth in mice.2 |
FAQs
What types of cancers can benefit from Creative Biolabs' Thymidine Kinase-loaded Oncolytic Adenovirus?
A: Our platform demonstrates promise in prostate, pancreatic, and other solid tumors, with a mechanism applicable to diverse malignancies. Its localized toxicity and systemic immunity induction enable versatile use. Discuss your cancer type and goals with our experts to determine the optimal strategy.
How does the "bystander effect" enhance the efficacy of this therapy?
A: The bystander effect is critical: toxic metabolites or signaling molecules from TK-expressing cells spread to neighboring non-infected cancer cells, killing them. This amplifies the oncolytic adenovirus' therapeutic reach, enabling widespread tumor destruction even with partial viral transduction-key to its robust efficacy.
Q: Is the adenoviral vector safe, especially considering potential immunogenicity or toxicity concerns?
A: Creative Biolabs ensures safety via advanced vector engineering. We use next-gen E1E4-deleted adenoviral vectors to reduce hepatotoxicity and minimize RCA contamination. This robust safety profile supports clinical translation.
Q: Can Creative Biolabs' oncolytic adenovirus platform be combined with other cancer treatments?
A: Our platform is designed for synergistic combinations with various therapies, such as traditional chemotherapy, radiation, and immunotherapies. A key strength is integrating immune-modulating genes (like sPD1-Ig or cytokines) into vectors, enabling multi-pronged cancer attacks for better outcomes.
Q: How can I monitor the effectiveness of the therapy in real-time during preclinical or clinical studies?
A: Our TK-loaded oncolytic adenovirus platform provides theragnostic capabilities. Using TK enzyme activity, advanced PET imaging tracks viral distribution and activity in real time, offering insights into treatment response for personalized adjustments.
Creative Biolabs leads in cancer gene therapy with a comprehensive platform of Thymidine Kinase-loaded Oncolytic Adenoviruses. Integrating scientific expertise and advanced vector engineering, our solutions deliver potent anti-tumor efficacy, immune activation, and high safety. Committed to translating research into therapeutic advancements, we help partners tackle oncology challenges for a cancer-curable future.
[Contact Our Team to Discuss Your Project]
Related Sections
| Cytosine Deaminase loaded Oncolytic Adenovirus | Prodrugs loaded Oncolytic Adenovirus |
| GM-CSF loaded Oncolytic Adenovirus | CD40L loaded Oncolytic Adenovirus |
| hNIS loaded Oncolytic Adenovirus | TNF-α loaded Oncolytic Adenovirus |
| IL-2 loaded Oncolytic Adenovirus | 41BBL loaded Oncolytic Adenovirus |
| PH20 Hyaluronidase loaded Oncolytic Adenovirus | Anti-CTLA4 loaded Oncolytic Adenovirus |
| Anti-PD1 loaded Oncolytic Adenovirus | IL-12 loaded Oncolytic Adenovirus |
| Decorin loaded Oncolytic Adenovirus | OX40L loaded Oncolytic Adenovirus |
| EGFR loaded Oncolytic Adenovirus | FRα loaded Oncolytic Adenovirus |
| FAP loaded Oncolytic Adenovirus | CD44v6 loaded Oncolytic Adenovirus |
References
- Zuo, Sipeng, et al. "Thymidine kinase 1 drives skin cutaneous melanoma malignant progression and metabolic reprogramming." Frontiers in Oncology 12 (2022): 802807. DOI: 10.3389/fonc.2022.802807. Distributed under Open Access license CC BY 4.0, without modification.
- Abate-Daga, Daniel, et al. "Oncolytic adenoviruses armed with thymidine kinase can be traced by PET imaging and show potent antitumoural effects by ganciclovir dosing." PLoS One 6.10 (2011): e26142. DOI: 10.1371/journal.pone.0026142. Distributed under Open Access license CC BY 4.0, the figures were cropped.
