Cytosine Deaminase loaded Oncolytic Adenovirus Engineering Service
Introduction
In oncology research, many faces pervasive challenges such as drug resistance, incomplete tumor eradication, and systemic toxicity. Our OncoVirapy™ Platform integrates engineered oncolytic adenoviruses with advanced prodrug activation systems to help overcome these therapeutic hurdles, achieving deeper and more durable responses through the combination of highly selective tumor destruction and localized potent drug activation.
[Discover How We Can Help - Request a Consultation]
Cytosine Deaminase-loaded Oncolytic Adenovirus
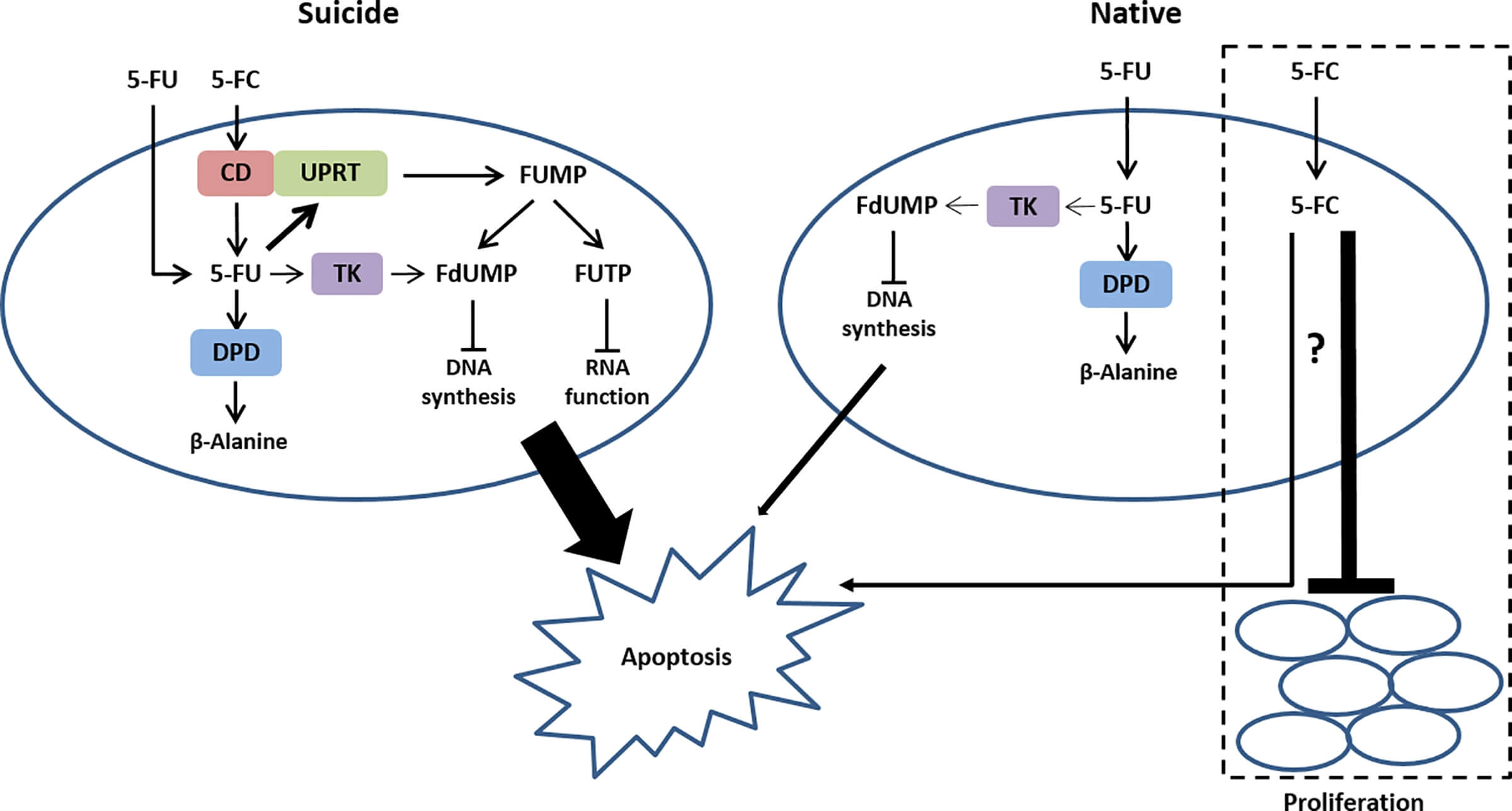 Fig.1 Mechanisms of 5-FC/5-FU and CD-related pro-apoptotic effects.1
Fig.1 Mechanisms of 5-FC/5-FU and CD-related pro-apoptotic effects.1
Cytosine Deaminase (CD) is a bacterial enzyme that plays a pivotal role in prodrug activation strategies within oncology. Unlike mammalian enzymes, CD is capable of efficiently converting the non-toxic antifungal prodrug 5-fluorocytosine (5-FC) into the potent chemotherapy agent 5-fluorouracil (5-FU). This distinct enzymatic activity allows for a highly specific therapeutic approach where the conversion, and thus the drug's cytotoxic effect, can be precisely localized.
Principle
The core principle of our services follows a two-step mechanism:
Viral Delivery & Gene Expression
- Engineered oncolytic adenovirus selectively infects and replicates in cancer cells.
- Directly lyses cancer cells while expressing the CD gene during replication.
Prodrug Activation & Bystander Effect
- Systemically administered non-toxic prodrug (5-FC) circulates in the body.
- CD enzyme in infected cancer cells converts 5-FC to cytotoxic 5-FU within the tumor microenvironment.
- 5-FU targets infected and nearby uninfected cancer cells via a "bystander effect," enhancing therapeutic impact.
Advantages
- Localized Drug Activation
Converts 5-FC to 5-FU primarily in tumors, reducing systemic 5-FU exposure and minimizing chemotherapy-related side effects.
- Enhanced Therapeutic Index
Localized action achieves higher drug concentrations at the tumor site with lower overall toxicity, improving the therapeutic index.
- Overcoming Drug Resistance
Targets hypoxic and resistant tumor cells; localized high 5-FU concentrations bypass traditional chemotherapy resistance, as shown in preclinical studies.
- Synergy with Existing Therapies
Compatible with radiotherapy or conventional chemo, enabling multi-modal strategies to enhance anti-tumor efficacy.
- Dual Mechanism of Action
Combines direct oncolytic viral lysis with locally generated cytotoxic 5-FU for a two-pronged attack on cancer cells.
Workflow
| Required Starting Materials | Project Consultation & Design |
|---|---|
|
Start with a detailed discussion to thoroughly understand your specific oncology challenge, therapeutic goals, and project scope. Have our expert team collaborate with you to design a customized gene-cell therapy strategy, outlining key milestones and expected outcomes to ensure alignment and a clear roadmap for success. |
| Adenovirus Engineering & Cytosine Deaminase Integration | In Vitro Validation & Optimization |
| Leverage cutting-edge molecular biology techniques to meticulously design and construct the oncolytic adenovirus vector. Incorporate the CD gene into the viral genome and optimize the construct for robust tumor selectivity and efficient replication in cancer cells while sparing healthy tissue. | Following engineering, conduct extensive cell culture experiments. Rigorously confirm viral tropism, oncolytic efficacy, prodrug conversion efficiency, and cytotoxicity in relevant cancer cell lines to refine the therapeutic approach. |
| Preclinical In Vivo Evaluation | Data Analysis & Reporting |
| Rigorously assess the engineered oncolytic adenovirus for therapeutic efficacy, biodistribution, and safety in relevant animal models. Test the adenovirus in combination with prodrug administration and standard-of-care therapies to observe synergistic effects and validate therapeutic potential in vivo. | Upon completing experimental phases, perform comprehensive analysis of all generated data-including molecular, cellular, and in vivo results. Then prepare detailed, transparent reports to summarize key findings, interpret their implications, and provide strategic recommendations for next steps, including clinical translation pathways. |
| Final Deliverables | Estimated Timeframe |
|
The typical timeframe for this service ranges from 10 to 15 weeks, depending on the complexity of the viral construct, the inclusion of optional preclinical evaluation, and the specific requirements of your project. More intricate designs or extensive in vivo studies may extend the duration. |
[Contact us to get more details]
What We Can Offer
At Creative Biolabs, our service is designed for precision and efficiency in oncology research. We understand each project's unique needs and offer fully customized solutions to match your scientific goals.
- Customized Viral Vector Design & Optimization: Tailored oncolytic adenovirus constructs with Cytosine Deaminase gene, optimized for specific tumors and therapeutic goals to maximize efficacy and specificity.
- Scalable Production of High-Titer Viral Vectors: Advanced bioprocessing for consistent high-titer, high-quality viral vectors, adaptable for preclinical/clinical development across project volumes.
- Comprehensive Preclinical Efficacy Assessment: Rigorous in vitro/in vivo studies to validate viral infectivity, oncolytic activity, prodrug conversion, and efficacy in cancer models.
- Robust Biosafety and Quality Control: Stringent biosafety measures with aseptic verification and QbD-based quality systems to ensure vector integrity and safety.
- Integrated Prodrug Strategy Development: Expert guidance on prodrug (e.g., 5-FC) administration protocols to optimize local activation, minimize systemic toxicity, and expand therapeutic window.
- Detailed Data Analysis and Regulatory Guidance: Comprehensive data packages, reports, and strategic support for navigating regulatory pathways toward clinical translation.
- Dedicated Scientific Collaboration: Close partnership with our scientific team throughout the project, offering continuous consultation and adaptive solutions for evolving research needs.
[Experience the Creative Biolabs Advantage - Get a Quote Today]
Case Study
The application of Cytosine Deaminase-loaded oncolytic adenoviruses in preclinical models and tumor cell lines enhances tumor-specific cytotoxicity. Studies confirm its potential for solid tumors, highlighting the virus's dual mechanism.
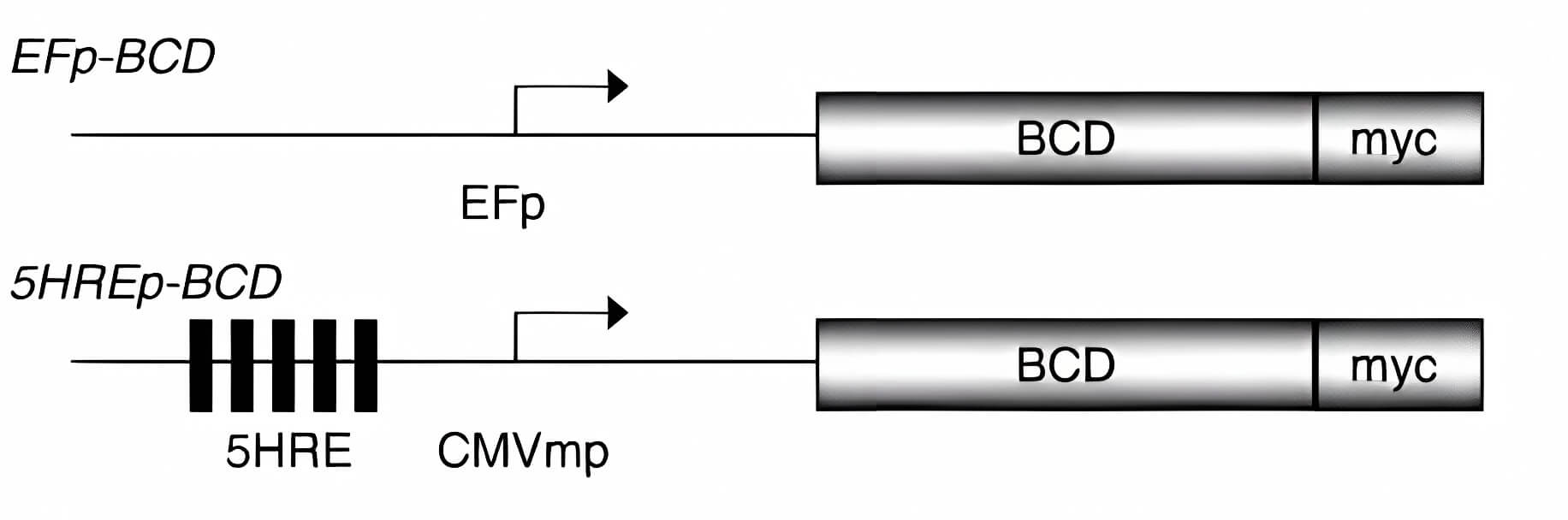
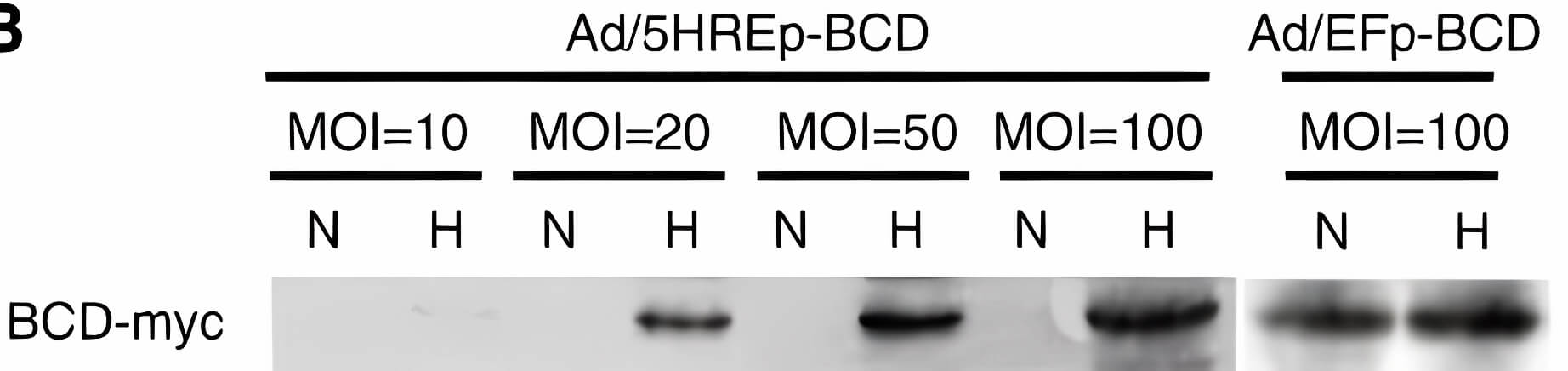
| Oncolytic Virus Construction | Expression of COX2 |
|---|---|
|
|
| Fig.2 Methods for genome construction of CD-loaded oncolytic adenovirus.2 | Fig.3 CD expression in tumor cells by CD-loaded oncolytic adenovirus.2 |
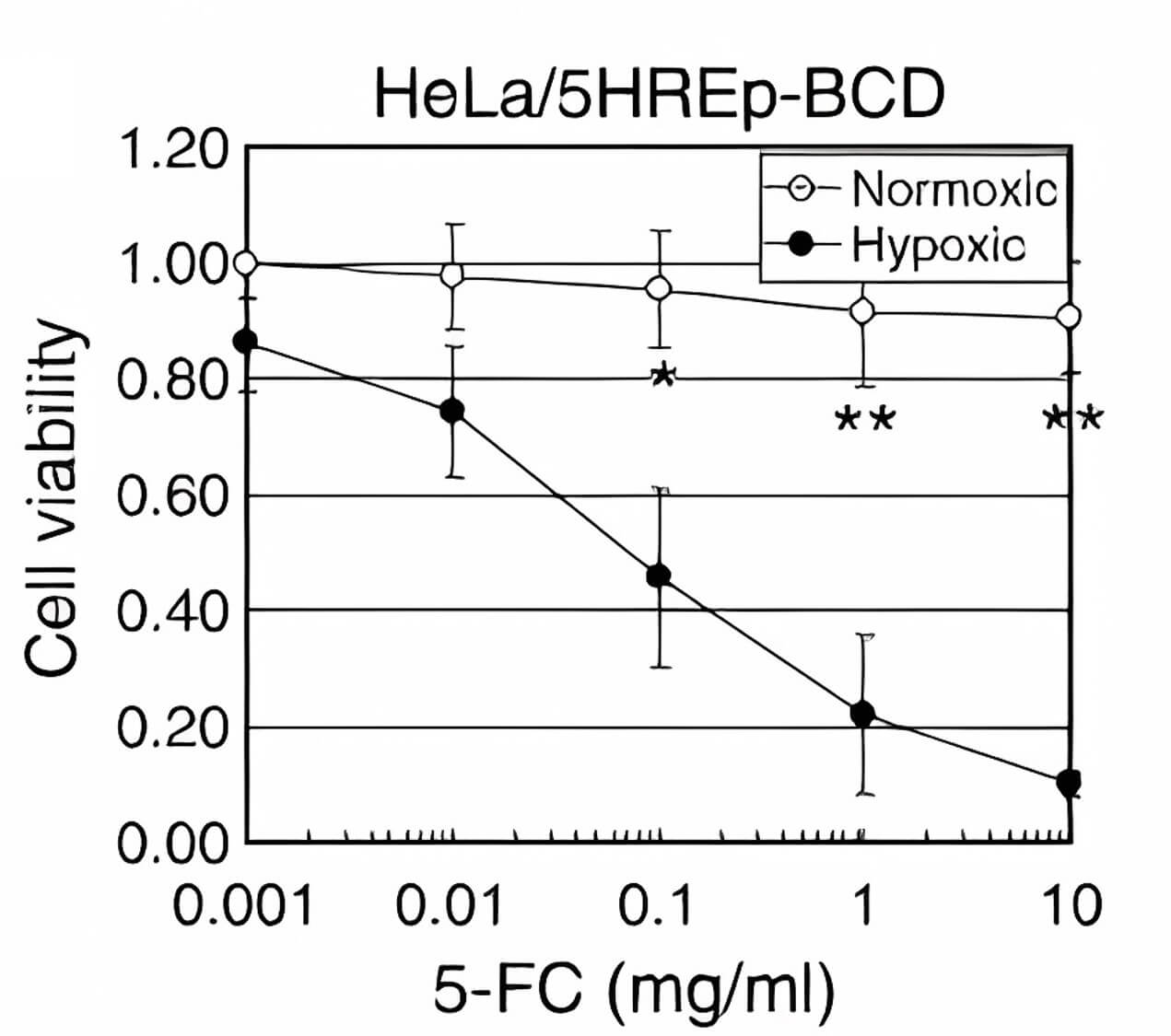
| Cytotoxicity | |
|
|
| Fig.4 Effect of CD-loaded oncolytic adenovirus on tumor cell activity.2 | |
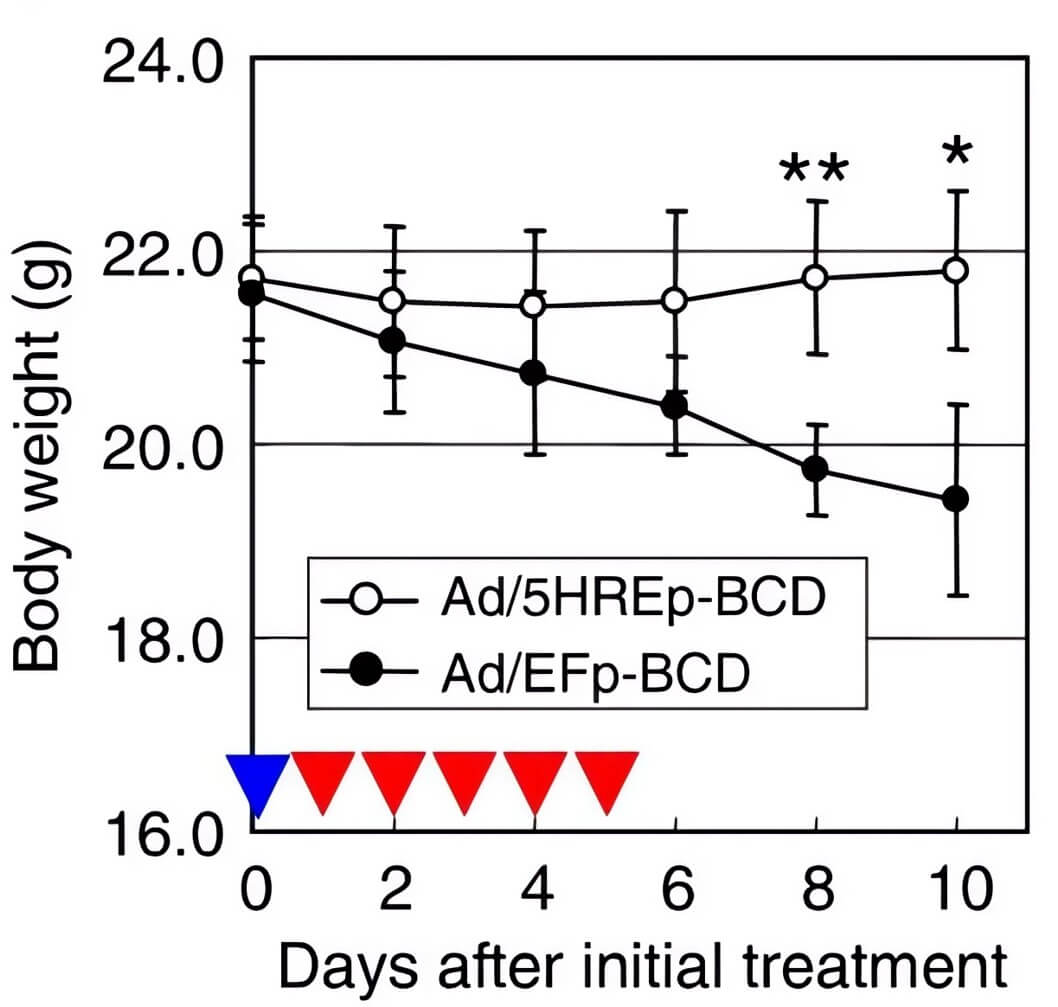
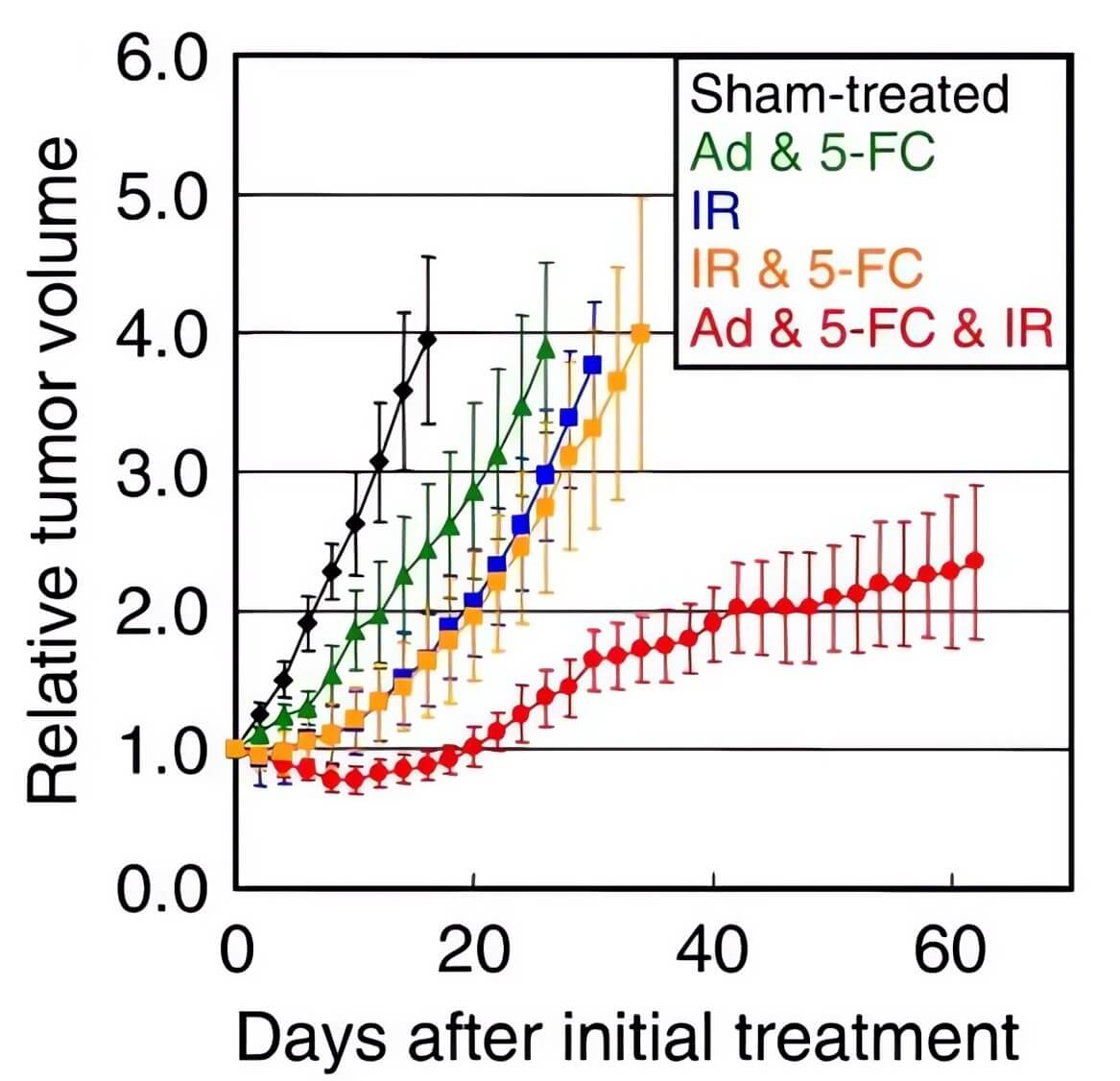
| Body Weight | Tumor Volume |
|
|
| Fig.5 CD-loaded oncolytic adenovirus could alleviate the weight loss of mice to a certain extent.2 | Fig.6 Oncolytic adenovirus loaded with CD can effectively attenuate tumor growth in mice when combined with other treatments.2 |
FAQs
How specific is the targeting of Cytosine Deaminase-loaded Oncolytic Adenoviruses to cancer cells?
A: Our oncolytic adenoviruses are engineered with tumor tropism and selective replication, infecting/replicating mainly in cancer cells to minimize off-target effects and boost efficacy/safety.
What types of cancers are most suitable for this approach?
A: This technology holds significant promise for a wide range of solid tumors, particularly those that exhibit inherent drug resistance, have challenging hypoxic microenvironments, or are difficult to treat with systemic therapies.
Can your service be integrated with our existing drug development pipeline?
A: Yes. Our service features flexibility and customization. We collaborate closely with your team to seamlessly integrate into your drug development pipeline, offering tailor-made solutions to complement your research and speed up clinical translation.
What are the key safety considerations for this therapy?
A: The Cytosine Deaminase-loaded Oncolytic Adenovirus boosts safety over traditional chemo: viral oncolysis self-limits to cancer cells, and localized prodrug activation generates chemo agent mainly at the tumor, cutting systemic side effects.
How does this technology compare to other gene therapy or targeted delivery methods?
A: Our technology delivers unique synergistic advantages by integrating direct oncolytic lysis with localized chemotherapeutic generation. This dual mechanism overcomes resistance and drives tumor regression more effectively than single-modality gene therapy or targeted delivery methods.
Related Sections
| Thymidine Kinase loaded Oncolytic Adenovirus | Prodrugs loaded Oncolytic Adenovirus |
| GM-CSF loaded Oncolytic Adenovirus | CD40L loaded Oncolytic Adenovirus |
| hNIS loaded Oncolytic Adenovirus | TNF-α loaded Oncolytic Adenovirus |
| IL-2 loaded Oncolytic Adenovirus | 41BBL loaded Oncolytic Adenovirus |
| PH20 Hyaluronidase loaded Oncolytic Adenovirus | Anti-CTLA4 loaded Oncolytic Adenovirus |
| Anti-PD1 loaded Oncolytic Adenovirus | IL-12 loaded Oncolytic Adenovirus |
| Decorin loaded Oncolytic Adenovirus | OX40L loaded Oncolytic Adenovirus |
| EGFR loaded Oncolytic Adenovirus | FRα loaded Oncolytic Adenovirus |
| FAP loaded Oncolytic Adenovirus | CD44v6 loaded Oncolytic Adenovirus |
References
- Gerace, Dario, et al. "Luciferase-based reporting of suicide gene activity in murine mesenchymal stem cells." Plos one 14.7 (2019): e0220013. DOI: 10.1371/journal.pone.0220013. Distributed under Open Access license CC BY 4.0, without modification.
- Liu, J., et al. "Adenovirus-mediated hypoxia-targeting cytosine deaminase gene therapy enhances radiotherapy in tumour xenografts." British journal of cancer 96.12 (2007): 1871-1878. DOI: 10.1038/sj.bjc.6603812. Distributed under Open Access license CC BY 4.0, the figures were cropped.
