GMCSF loaded Oncolytic Adenovirus Engineering Service
Introduction
Many researchers face challenges like limited efficacy in conventional cancer therapies, immunosuppressive tumor microenvironments, or difficulties in generating durable anti-tumor immunity. Our GMCSF-loaded Oncolytic Adenovirus service engineers dual-action therapeutics via advanced virotherapy and immunomodulation to directly destroy cancer cells while stimulating robust, tumor-specific immune responses. OncoVirapy™ Platform delivers tailored, highly effective GMCSF-loaded oncolytic adenoviruses to target/lyse tumor cells, re-educate the microenvironment, enhance therapeutic windows, overcome resistance, and establish long-term immune memory for solid tumors-accelerating your oncology pipeline from concept to preclinical validation.
[Discover How We Can Help - Request a Consultation]
GMCSF-loaded Oncolytic Adenovirus
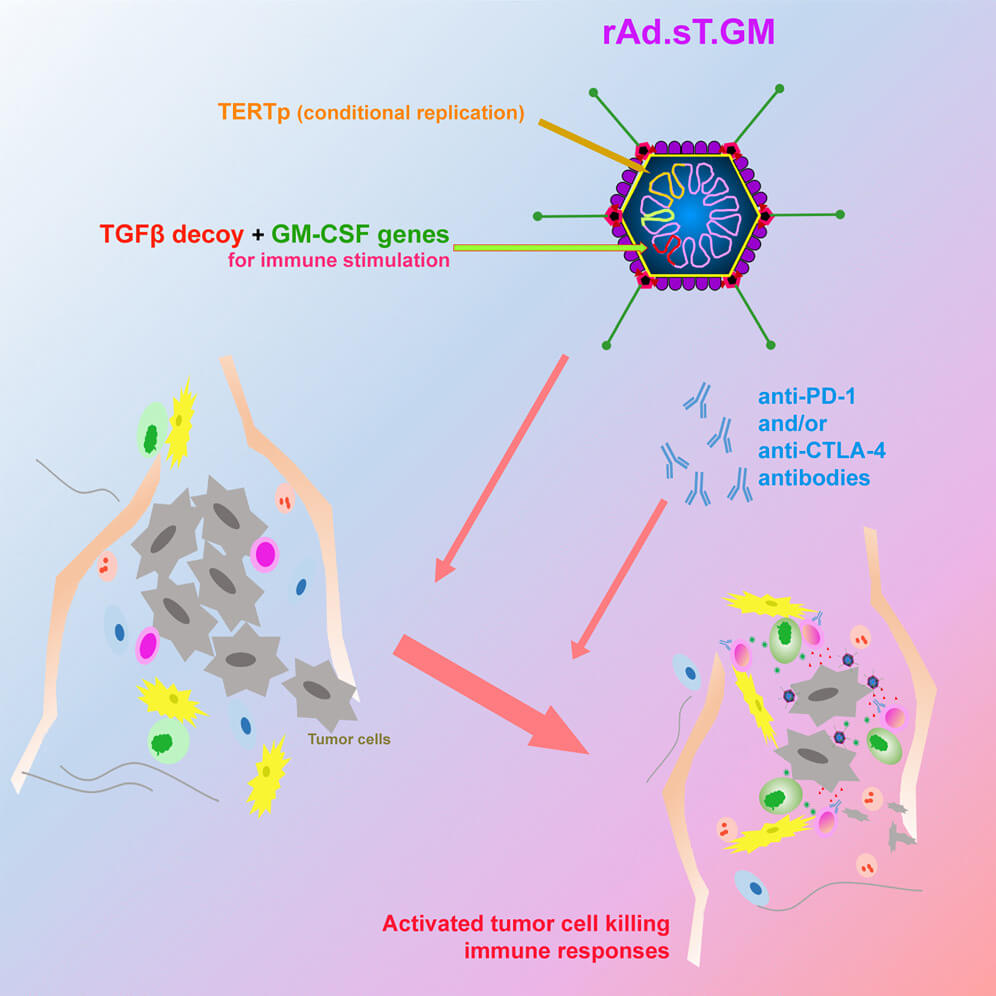 Fig.1 Oncolytic adenovirus inhibits tumor growth or metastasis by overexpressing GM-CSF.1
Fig.1 Oncolytic adenovirus inhibits tumor growth or metastasis by overexpressing GM-CSF.1
Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF) is a pleiotropic cytokine renowned for its critical role in hematopoiesis and immune cell regulation. In the context of cancer immunotherapy, GM-CSF acts as a powerful recruiter and activator of antigen-presenting cells (APCs), particularly dendritic cells. By locally delivering GM-CSF directly into the tumor microenvironment via an oncolytic adenovirus, we can orchestrate a profound immunological shift, transforming an immunosuppressive milieu into one conducive to effective anti-tumor immunity.
Principle
- Direct Oncolysis: The engineered adenovirus with deletions (e.g., E1A region) selectively replicates in cancer cells with defective tumor suppressor pathways (e.g., Rb pathway), leading to lysis and release of DAMPs (danger-associated molecular patterns) and tumor antigens.
- Immune Priming and Amplification: Viral replication triggers local GM-CSF secretion within the tumor microenvironment, chemoattracting and activating dendritic cells and other antigen-presenting cells (APCs). Activated APCs capture tumor antigens, migrate to lymph nodes, and prime tumor-specific cytotoxic T lymphocytes (CTLs), which systemically target primary and metastatic lesions.
Advantages
- Enhanced Anti-Tumor Immunity: GM-CSF promotes recruitment/activation of antigen-presenting cells, enhancing tumor antigen presentation and boosting targeted T-cell responses.
- Systemic Efficacy: Stimulates systemic immunity to target distant metastases and prevent recurrence-critical for advanced cancer.
- Overcoming Immunosuppression: Oncolysis combined with GM-CSF delivers "danger signals" to disrupt tumor immune tolerance and override microenvironmental immunosuppression.
- Synergy with Other Immunotherapies: Immune-modulating effects prime tumors for checkpoint inhibitors and other immunotherapies, improving combination therapy outcomes.
- Targeted Delivery: Local GM-CSF production in tumors reduces systemic cytokine exposure, minimizing off-target side effects of systemic GM-CSF administration.
Workflow
| Required Starting Materials | Project Consultation & Design |
|---|---|
|
Our expert team engages in in-depth discussions to understand your project's unique requirements, biological targets, and desired applications. We assess the feasibility and outline a strategic development plan. |
| Oncolytic Virus Engineering & Arming | In Vitro Validation & Characterization |
| Using state-of-the-art recombinant DNA technology, engineer the oncolytic adenovirus backbone with high precision to ensure tumor selectivity. Simultaneously, insert the human GMCSF gene to ensure robust, replication-dependent expression in infected tumor cells. Stringent quality control measures verify genetic integrity. | Oncolytic effects were evaluated and GMCSF expression/function was quantified by performing numerous in vitro assays. This includes assessment of viral transmission kinetics and dose-response relationships. |
| Preclinical In Vivo Efficacy Assessment | Regulatory Strategy & GMP-Ready Manufacturing Support |
| The engineered virus undergoes rigorous evaluation in immunocompetent animal models (e.g., syngeneic hamster models for semi-permissive human adenovirus studies) to assess in vivo tumor regression, systemic dissemination, safety parameters | Experienced teams provide expert guidance for the regulatory pathway, assisting with packet preparation for the pre-IND meeting. They can also support the transition to Good Manufacturing Practice (GMP) -compliant manufacturing, ensuring scalability and consistency in clinical trials. |
| Final Deliverables | Estimated Timeframe |
|
The typical timeframe for this service ranges from 10 to 15 weeks, depending on the complexity of the viral construct, the inclusion of optional preclinical evaluation, and the specific requirements of your project. More intricate designs or extensive in vivo studies may extend the duration. |
What we can offer
At Creative Biolabs, we empower biology experts like you with bespoke solutions for advancing your oncolytic virotherapy projects. Our service is designed to seamlessly integrate into your research and development pipeline, offering unparalleled flexibility and quality. We provide:
One-stop Development and Manufacturing
Comprehensive integrated service from early-stage vector design and lab-scale R&D to pilot/large-scale clinical-grade production of GMCSF-loaded oncolytic adenoviruses.
Efficient Process Optimization
Tailored upstream/downstream process development for maximal viral vector yield, purity, and functional integrity.
Scalable Production Capabilities
Flexible robust manufacturing to meet diverse project needs, ensuring seamless transition from research to large-scale clinical supply.
Integrated Quality Systems
Implementation of Quality-by-Design (QbD) and Process Analytical Technologies (PAT) throughout the development/manufacturing lifecycle.
Rigorous Aseptic Control
Strict adherence to aseptic verification and environmental monitoring during production to guarantee product safety/purity.
GMP-Compliant Manufacturing
Production under stringent Good Manufacturing Practice (GMP) guidelines for regulatory compliance and clinical readiness.
Guaranteed Vector Stability
Systematic assessment and documentation to ensure long-term stability of viral seed banks and final products.
Optimized Gene Expression
Advanced genetic engineering (e.g., expression cassette optimization) for robust functional GMCSF expression in oncolytic adenoviruses.
Flexible Production Modalities
Expertise in batch/fed-batch processes to optimize culture conditions and maximize product yield/quality.
High-Standard Quality Control
Comprehensive rigorous QC assays using advanced tools for precise quantification and exhaustive product characterization.
[Experience the Creative Biolabs Advantage - Get a Quote Today]
Case Study
The deployment of GMCSF-loaded oncolytic adenoviruses in preclinical setups and tumor cell lineages amplifies tumor-selective cytolytic potency. Inquiries corroborate its feasibility for solid neoplasms, emphasizing the virus's dual-mechanism paradigm.
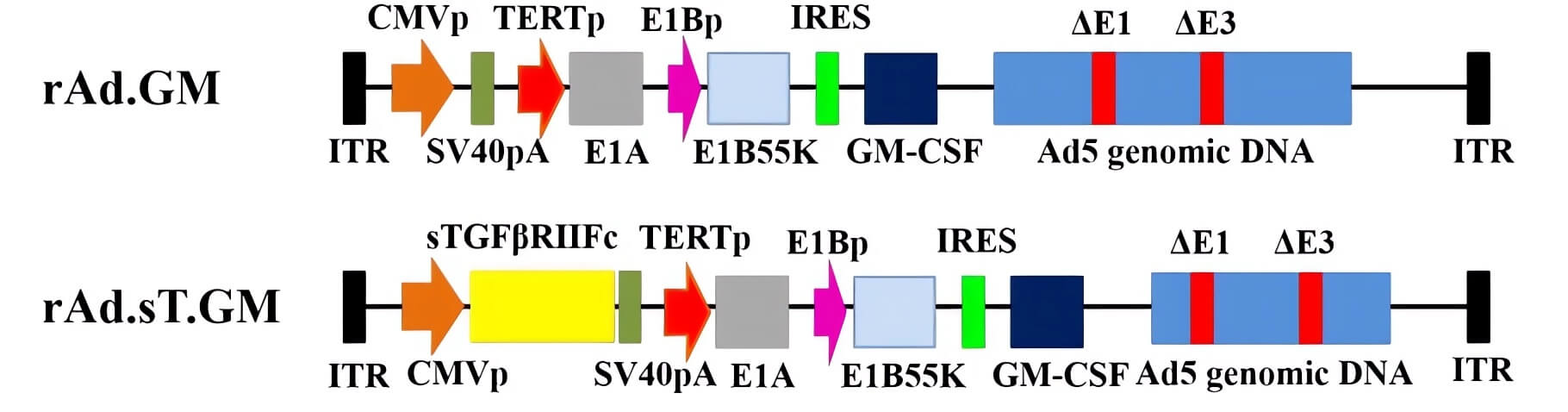
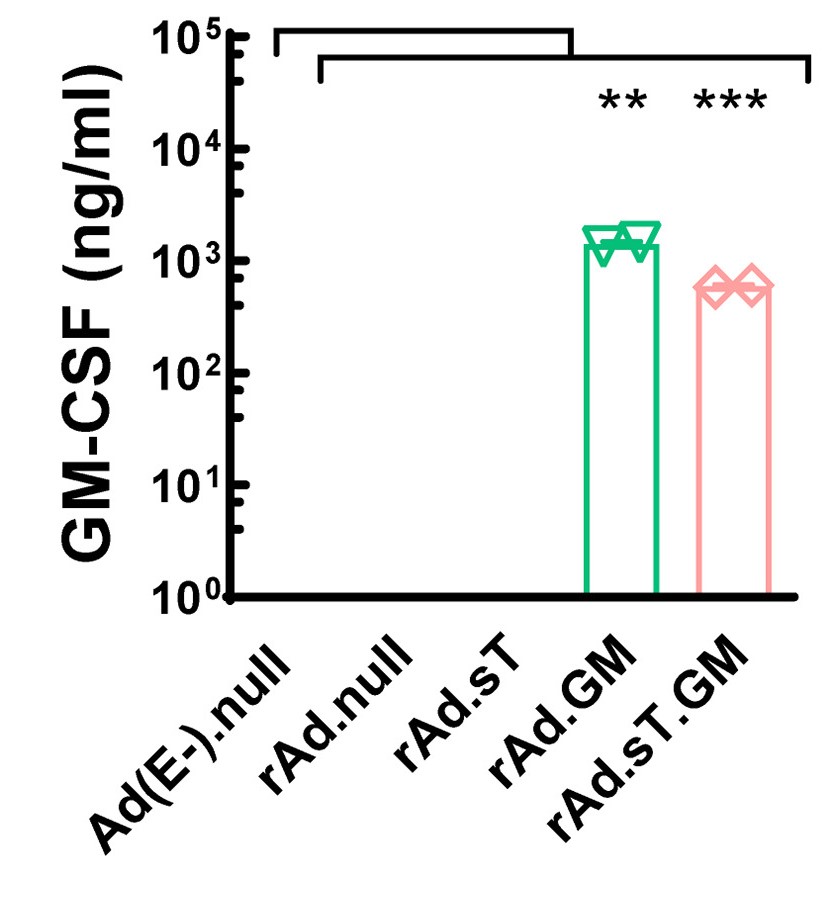
| Oncolytic Virus Construction | Expression of GM-CSF |
|---|---|
|
|
| Fig.2 The genome composition of oncolytic adenovirus loaded with GMCSF is constructed.1 | Fig.3 The oncolytic adenovirus loaded with GMCSF can express GMCSF in tumor cells.1 |
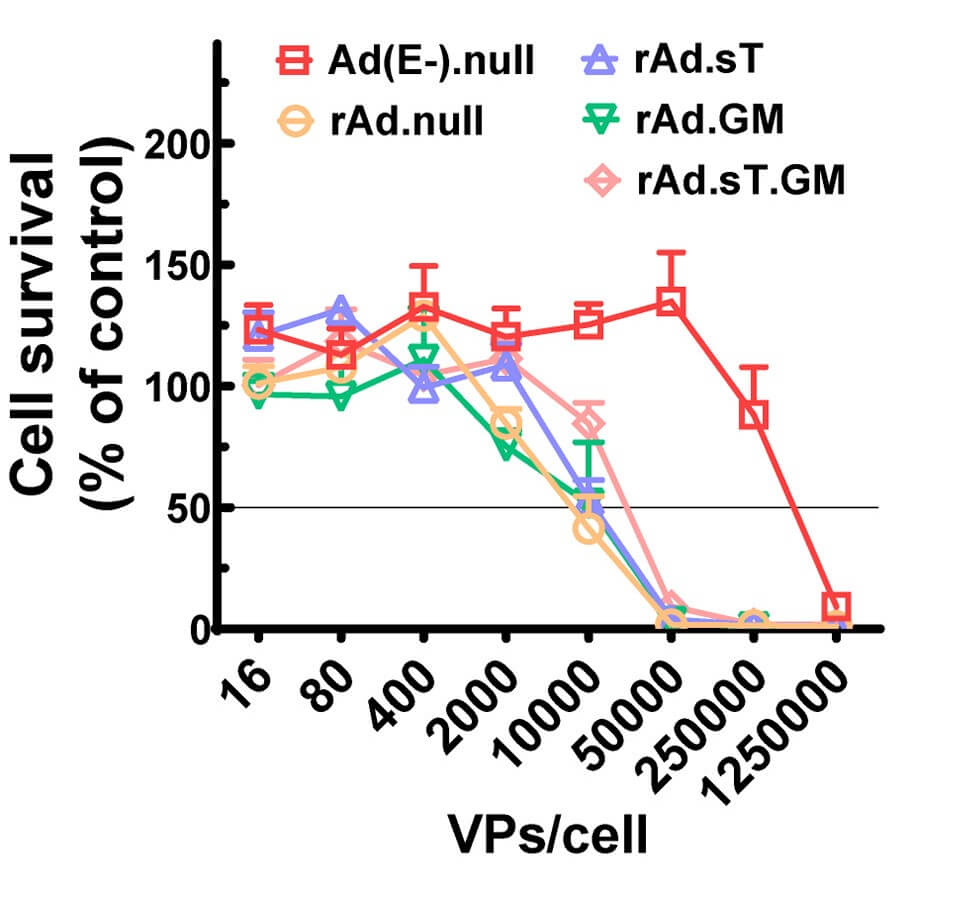
| Cell Viability | Tumor Volume |
|
|
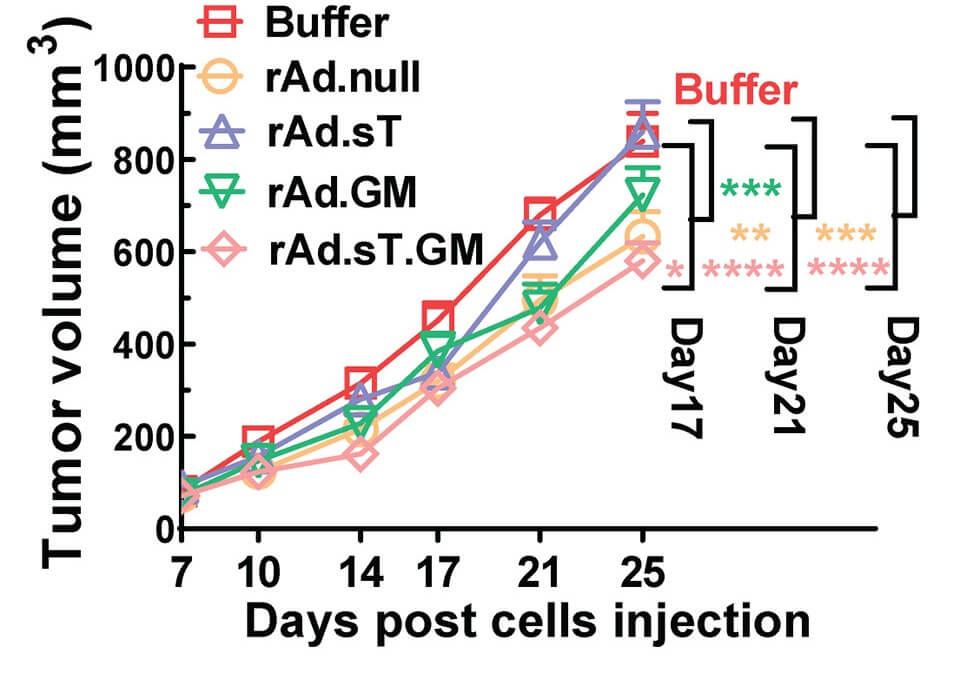
| Fig.4 Oncolytic adenovirus loaded with GMCSF could reduce tumor cell activity to some extent.1 | Fig.5 Oncolytic adenovirus loaded with GMCSF slowed tumor growth in tumor-bearing mice.1 |
| Tumor Weight | Tumor GM-CSF |
|
|
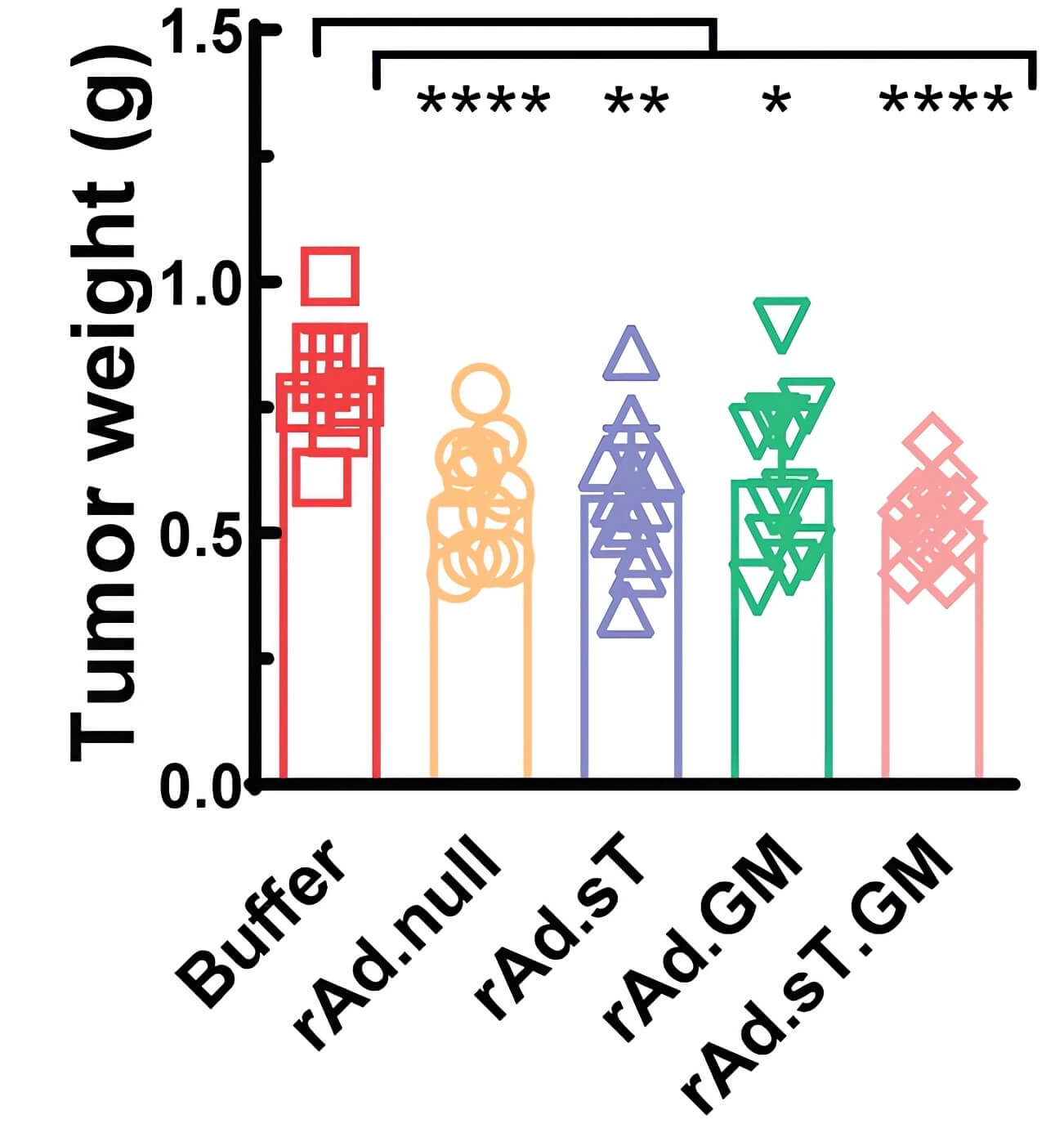
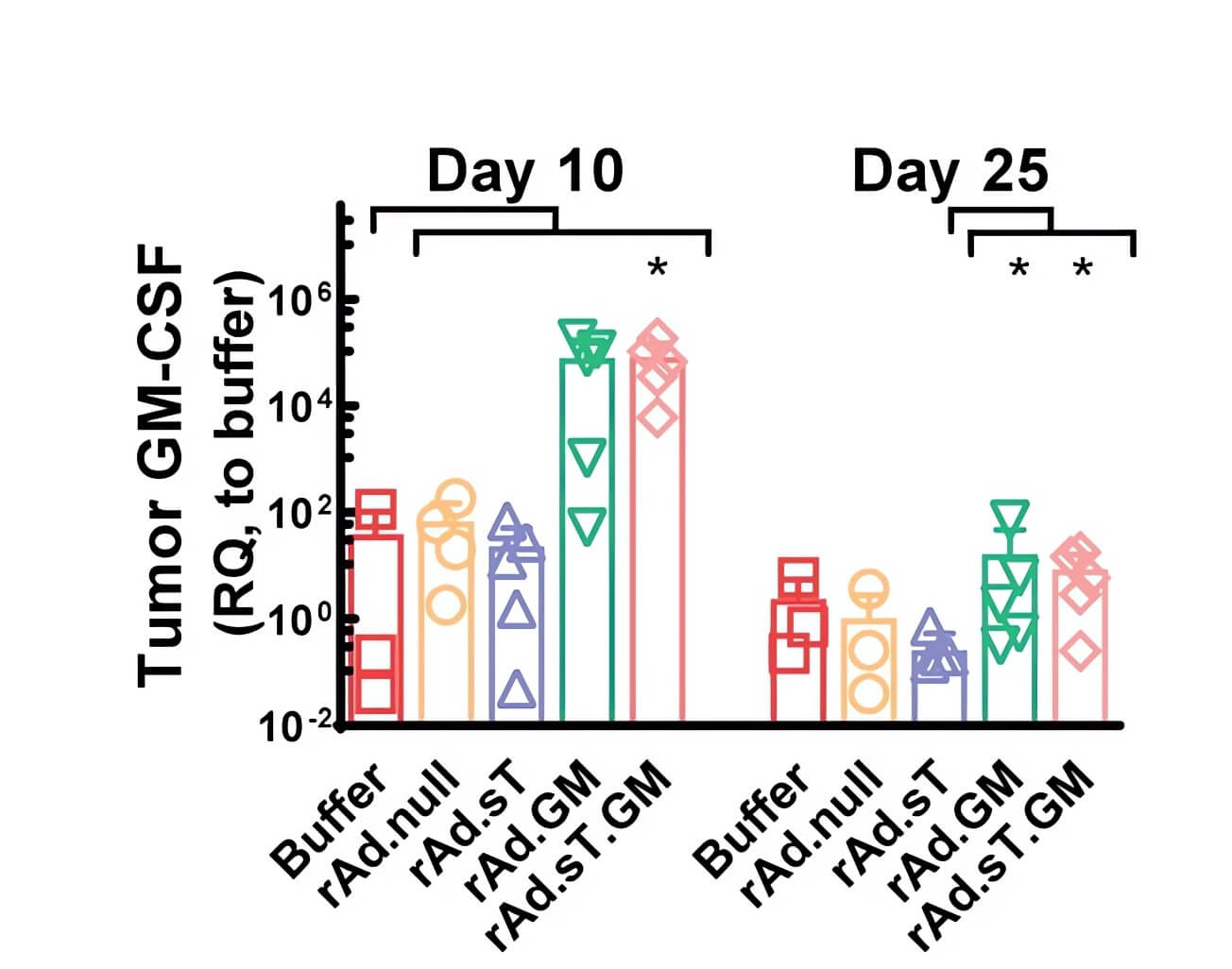
| Fig.6 Tumor weight was significantly reduced in mice treated with oncolytic adenovirus loaded with GMCSF.1 | Fig.7 The expression level of GMCSF in tumor cells of mice treated with oncolytic adenovirus loaded with GMCSF was significantly higher than that of other groups.1 |
FAQs
Q1: How does Creative Biolabs ensure the tumor selectivity of its GMCSF-loaded oncolytic adenoviruses, and is it truly safe for normal tissues?
A1: Our oncolytic adenoviruses feature genetic modifications for cancer-selective replication, exploiting tumor suppressor pathway defects. Normal cells remain spared due to intact pathways. Preclinical/clinical data show favorable safety profiles with minimal healthy tissue impact.
Q2: What types of cancer can potentially be treated with GMCSF-loaded oncolytic adenoviruses, and how does this approach compare to traditional chemotherapy or radiation?
A2: GMCSF-loaded oncolytic adenoviruses demonstrate potential in diverse solid tumors, particularly those with immunosuppressive microenvironments or a low response to conventional therapies. Unlike chemotherapy and radiation, which can cause systemic toxicity and weak immune activation, this dual-action approach targets cancer cells directly while priming the immune system to recognize primary and metastatic tumors, enabling durable systemic anti-tumor immunity.
Q3: How does the GMCSF-loaded oncolytic adenovirus enhance the patient's immune response, and what specific immune cells are involved?
A3: The oncolytic adenovirus lyses tumor cells, releasing antigens and danger signals. Encoded GM-CSF recruits/activates APCs (e.g., dendritic cells), which process antigens and prime tumor-specific CD8+ T cells. Activated T cells infiltrate tumors for targeted killing. GM-CSF also directly activates natural killer cells to enhance anti-tumor effects.
Q4: What is the typical administration method for GMCSF-loaded oncolytic adenoviruses, and can it target metastatic disease effectively?
A4: Intratumoral injection enables direct viral delivery for local replication and GM-CSF production, while triggering systemic immune responses. Tumor-specific T cells migrate to eliminate distant metastases. We design optimized administration strategies tailored to your tumor model and clinical goals for maximal systemic efficacy.
Q5: What support does Creative Biolabs provide for advancing my GMCSF-loaded oncolytic adenovirus project from preclinical development towards clinical trials?
A5: Creative Biolabs provides end-to-end support from early research to regulatory readiness, including preclinical validation, safety/efficacy reporting, and IND strategy guidance. We help craft compelling data packages for clinical advancement—contact us to streamline your translational path.
Creative Biolabs offers end-to-end solutions for GMCSF-loaded oncolytic adenovirus development, covering virus engineering, preclinical validation, and regulatory support. Our platform delivers innovative therapies with enhanced tumor selectivity, immune activation, and systemic anti-tumor efficacy for advanced cancer treatment. Partner with us to accelerate immuno-oncology breakthroughs.
Contact Our Team to Discuss Your Project!
Related Sections
| Cytosine Deaminase loaded Oncolytic Adenovirus | Thymidine Kinase loaded Oncolytic Adenovirus |
| Progrugs loaded Oncolytic Adenovirus | CD40L loaded Oncolytic Adenovirus |
| hNIS loaded Oncolytic Adenovirus | TNF-α loaded Oncolytic Adenovirus |
| IL-2 loaded Oncolytic Adenovirus | 41BBL loaded Oncolytic Adenovirus |
| PH20 Hyaluronidase loaded Oncolytic Adenovirus | Anti-CTLA4 loaded Oncolytic Adenovirus |
| Anti-PD1 loaded Oncolytic Adenovirus | IL-12 loaded Oncolytic Adenovirus |
| Decorin loaded Oncolytic Adenovirus | OX40L loaded Oncolytic Adenovirus |
| EGFR loaded Oncolytic Adenovirus | FRα loaded Oncolytic Adenovirus |
| FAP loaded Oncolytic Adenovirus | CD44v6 loaded Oncolytic Adenovirus |
Reference
- Nhàn, Nguyễn Thị Thanh, et al. "Oncolytic adenovirus inhibits TNBC tumor growth/metastasis in mice by targeting TGFB and overexpressing GM-CSF." Molecular Therapy Oncology 33.1 (2025): 200936. DOI: 10.1016/j.omton.2025.200936. Distributed under Open Access license CC BY 4.0, some figures were cropped.
