Prodrug loaded Oncolytic Adenovirus Engineering Service
Introduction
Our service uses VDEPT and precision engineering for targeted tumor destruction. Creative Biolabs provides tailored solutions, engineering oncolytic adenoviruses to carry prodrug-activating enzymes that convert non-toxic prodrugs to cytotoxins in the tumor microenvironment. This minimizes systemic side effects, improves therapeutic index, and enhances efficacy via localized cell death and immune stimulation.
[Discover How We Can Help - Request a Consultation]
Prodrugs-loaded Oncolytic Adenovirus
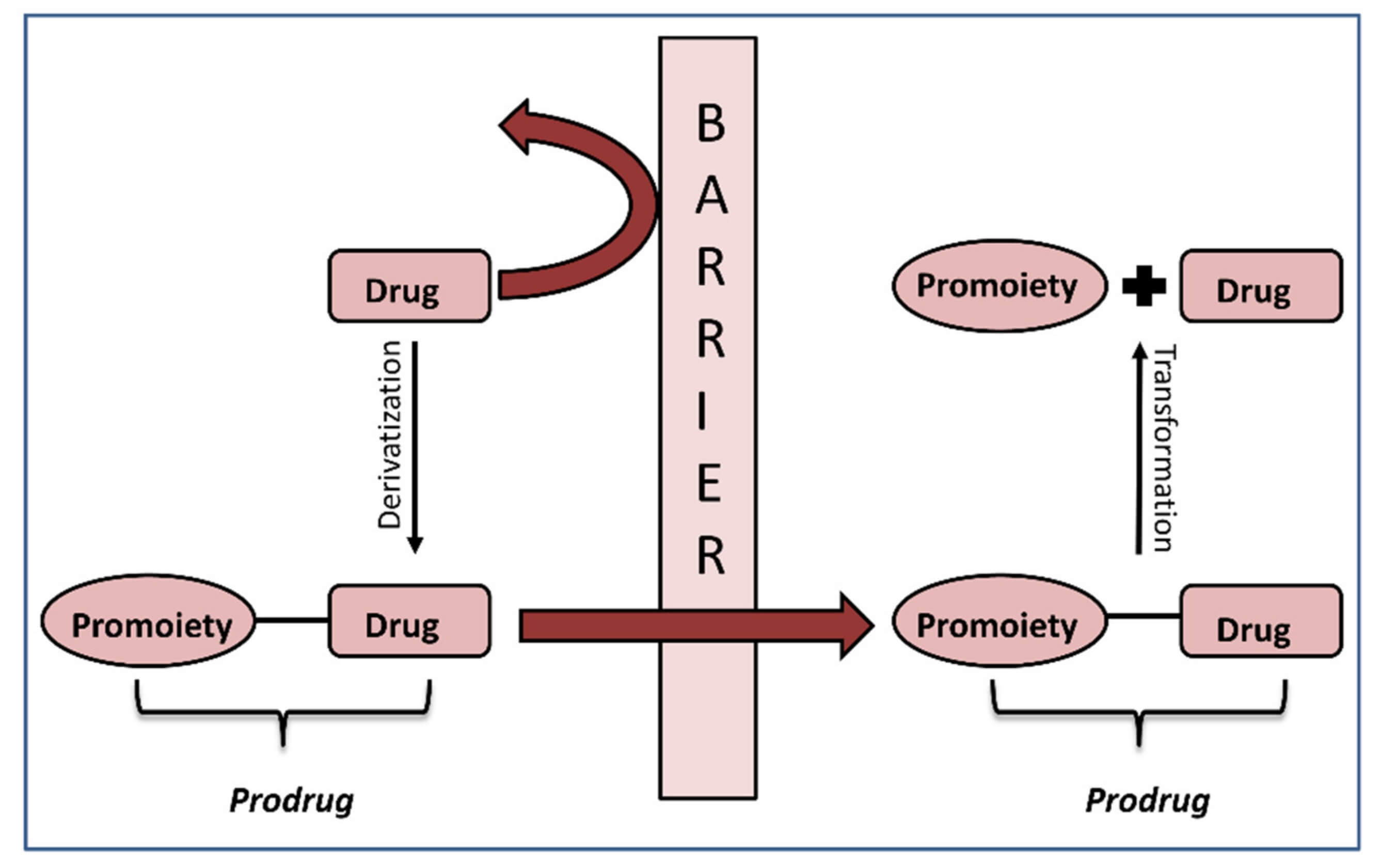 Fig.1 The concept of prodrugs is explained.1
Fig.1 The concept of prodrugs is explained.1
Prodrugs are pharmacologically inactive compounds that, upon administration, are metabolized in vivo to their active drug form. In the context of cancer therapy, bio reductive prodrugs are specifically designed to be activated under the hypoxic (low oxygen) conditions prevalent in solid tumors, or by enzymes highly expressed within cancer cells. This design principle allows for localized drug activation, minimizing systemic exposure to the potent cytotoxic agent and thereby reducing off-target side effects commonly associated with conventional chemotherapy. Examples include dinitrobenzamide mustards (DNBMs) like PR-104, which are activated by enzymes such as nitroreductases (NTRs) into highly DNA-damaging metabolites.
Core Principle
- Prodrugs-loaded oncolytic adenovirus therapy relies on tumor-specific activation of therapeutic agents:
- Genetically engineered oncolytic adenoviruses replicate selectively in cancer cells.
- Viral genomes carry genes for enzymes (e.g., nitroreductase, cytosine deaminase), which are expressed during replication.
- Enzymes convert non-toxic prodrugs into cytotoxic forms, killing infected cancer cells.
- Activated cytotoxins exhibit a "bystander effect," diffusing to kill neighboring uninfected tumor cells.
Advantages
- Enhanced Therapeutic Index: Localizing cytotoxic activation to tumors reduces systemic toxicity, enabling higher intratumoral drug concentrations for improved efficacy with fewer side effects.
- Improved Tumor Distribution and Viral Titre: Studies show prodrugs increase viral titre and tumor distribution, overcoming non-uniform spread in solid tumors.
- Dual Mechanism of Action: Combines direct oncolysis (viral lysis), enzyme-mediated chemotherapy, and anti-tumor immune stimulation. Oncolysis releases antigens to prime long-term immunity.
- Circumvention of Antiviral Resistance: Carefully selected prodrug-enzyme pairs show no antiviral effects at therapeutic doses, ensuring continuous viral replication.
- Targeting Tumor Heterogeneity: Bystander effect of activated cytotoxins diffuses to uninfected/hypoxic tumor cells, addressing inherent tumor heterogeneity.
- Synergy with Immunotherapy: Local cytotoxicity and neoantigen release create a pro-inflammatory microenvironment, amplifying the efficacy of immune checkpoint inhibitors for durable responses.
Workflow
| Required Starting Materials | Project Consultation & Design |
|---|---|
|
Engage in in-depth discussions with our expert team to clarify your specific research objectives, therapeutic targets, and desired outcomes. Review your provided materials and relevant background information. |
| Vector Engineering & Optimization | Prodrug System Characterization & Selection |
| Genetic modification of oncolytic adenovirus vectors to incorporate genes for specific prodrug-activating enzymes. This involves cloning, viral propagation, and purification. | In-depth in vitro analysis of the selected prodrugs in combination with the engineered viruses. This includes cytotoxicity assays, assessment of prodrug activation kinetics, and evaluation of bystander effects in 2D and 3D cell culture models. |
| In Vitro Efficacy Assessment & Antiviral Effects | In Vivo Preclinical Evaluation |
| Rigorous testing of the oncolytic virus-prodrug combination in various in vitro models. We evaluate viral replication kinetics in the presence of activated prodrugs to ensure no adverse antiviral effects, and assess the therapeutic window. | Moving to in vivo models, typically using immunocompromised or syngeneic mouse xenograft models. This involves systemic or intratumoral administration of the virus and prodrug, followed by monitoring tumor growth, viral distribution, and prodrug metabolism. |
| Final Deliverables | Estimated Timeframe |
|
The typical timeframe for this service ranges from 10 to 15 weeks, depending on the complexity of the viral construct, the inclusion of optional preclinical evaluation, and the specific requirements of your project. More intricate designs or extensive in vivo studies may extend the duration. |
[Contact Us to Get More Information]
What We Can Offer
At Creative Biolabs, we offer a robust suite of services and products designed to accelerate your research into Prodrugs-loaded Oncolytic Adenoviruses. As a leader in this cutting-edge field, we provide customized solutions to meet the unique demands of your oncology projects, delivering high-quality data and actionable insights that drive therapeutic innovation.
- Customized Viral Vector Design & Engineering
Tailored genetic modification of oncolytic adenoviruses to express prodrug-activating enzymes (e.g., NTR, CD) with optimized tumor selectivity and expression, ensuring safe/effective therapeutic payload delivery.
- Comprehensive Prodrug Characterization
In-depth in vitro evaluation of prodrugs via 2D/3D tumor models, analyzing activation kinetics, cytotoxicity, and bystander effects to screen potent/safe candidates.
- Advanced Preclinical Efficacy Studies
Rigorous in vivo testing using diverse tumor models to assess tumor growth inhibition, viral distribution/titre, and in situ prodrug metabolism for development decisions.
- Integrated PK/PD Analysis
Comprehensive assessment of prodrug pharmacokinetics/pharmacodynamics in the tumor microenvironment to optimize dosing and confirm localized activation.
- Synergy with Immunotherapy Assessment
Evaluation of immune-stimulatory effects (e.g., immune cell infiltration, cytokine profiles) to explore synergies with checkpoint inhibitors and other immunotherapies.
- Robust Quality Control & Assurance
Adherence to highest quality standards throughout viral production and data analysis to ensure reproducibility, reliability, and regulatory compliance.
[Experience the Creative Biolabs Advantage - Get a Quote Today!]
Case Study
The employment of Prodrugs-loaded oncolytic adenoviruses in preclinical frameworks and tumor cellular systems elevates tumor-specific cytocidal activity. Researches authenticate its viability for solid tumors, accentuating the virus's twofold mechanistic approach.
| Cell Viability | Cytotoxicity |
|---|---|
|
|
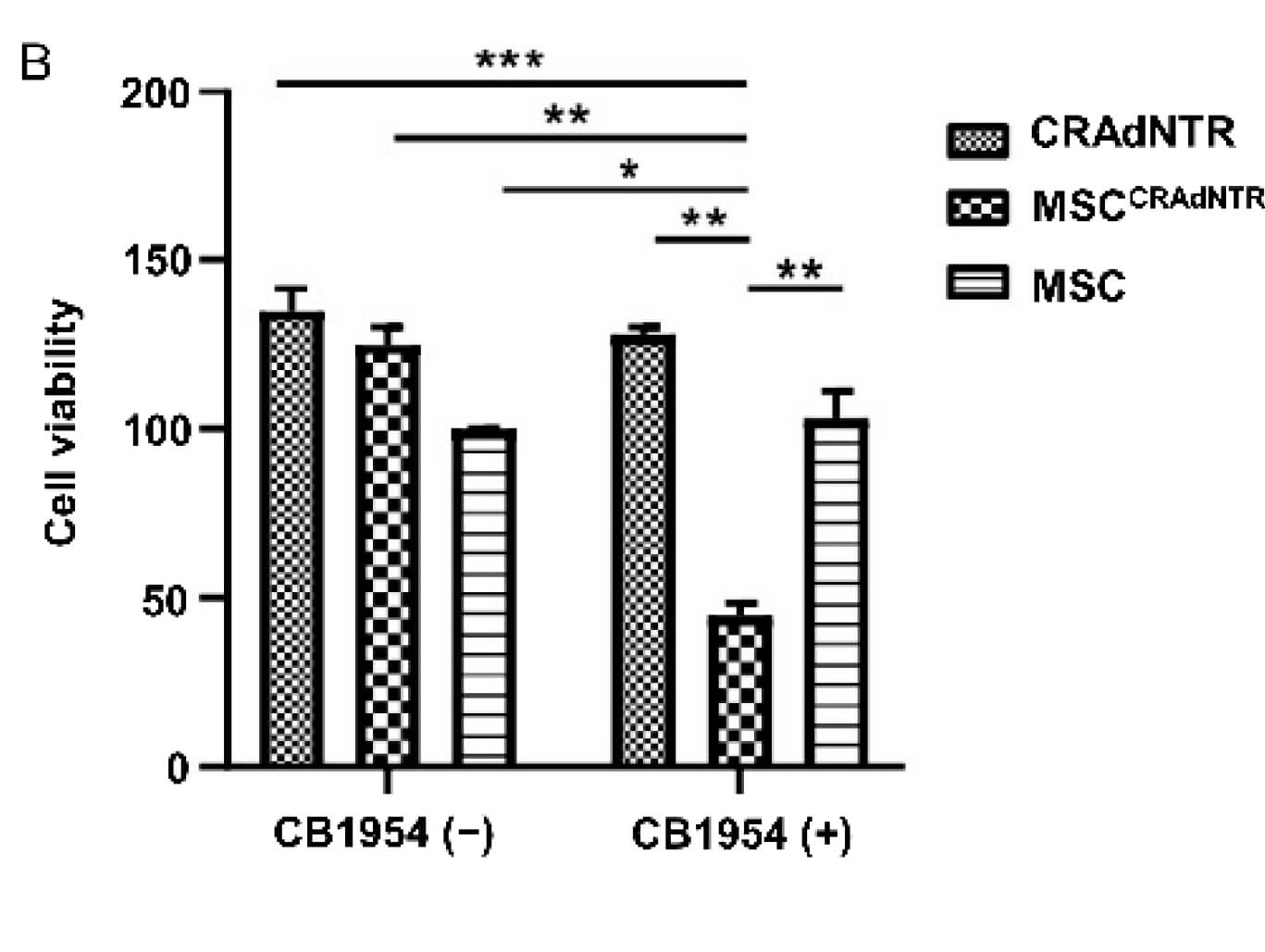
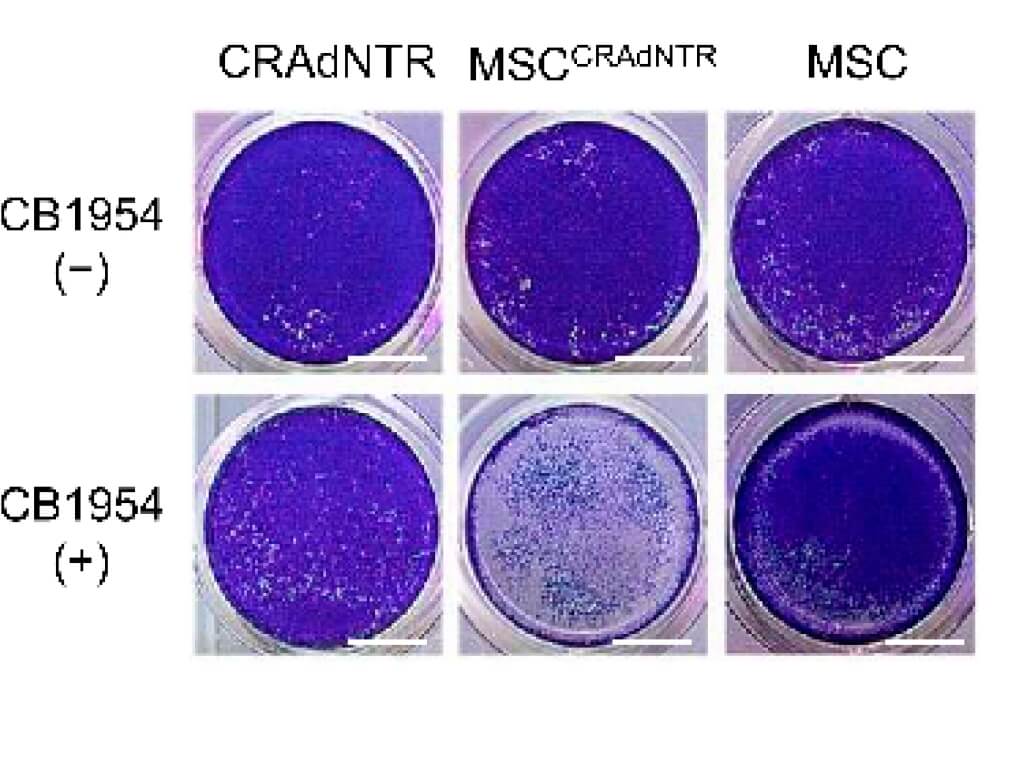
| Fig.2 MTT assay is used to detect the effect of oncolytic adenovirus on the activity of tumor cells.2 | Fig.3 Crystal violet staining was used to observe the cytotoxicity of oncolytic adenovirus loaded with prodrug on tumor cells.2 |
| Tumor Volume | |
|
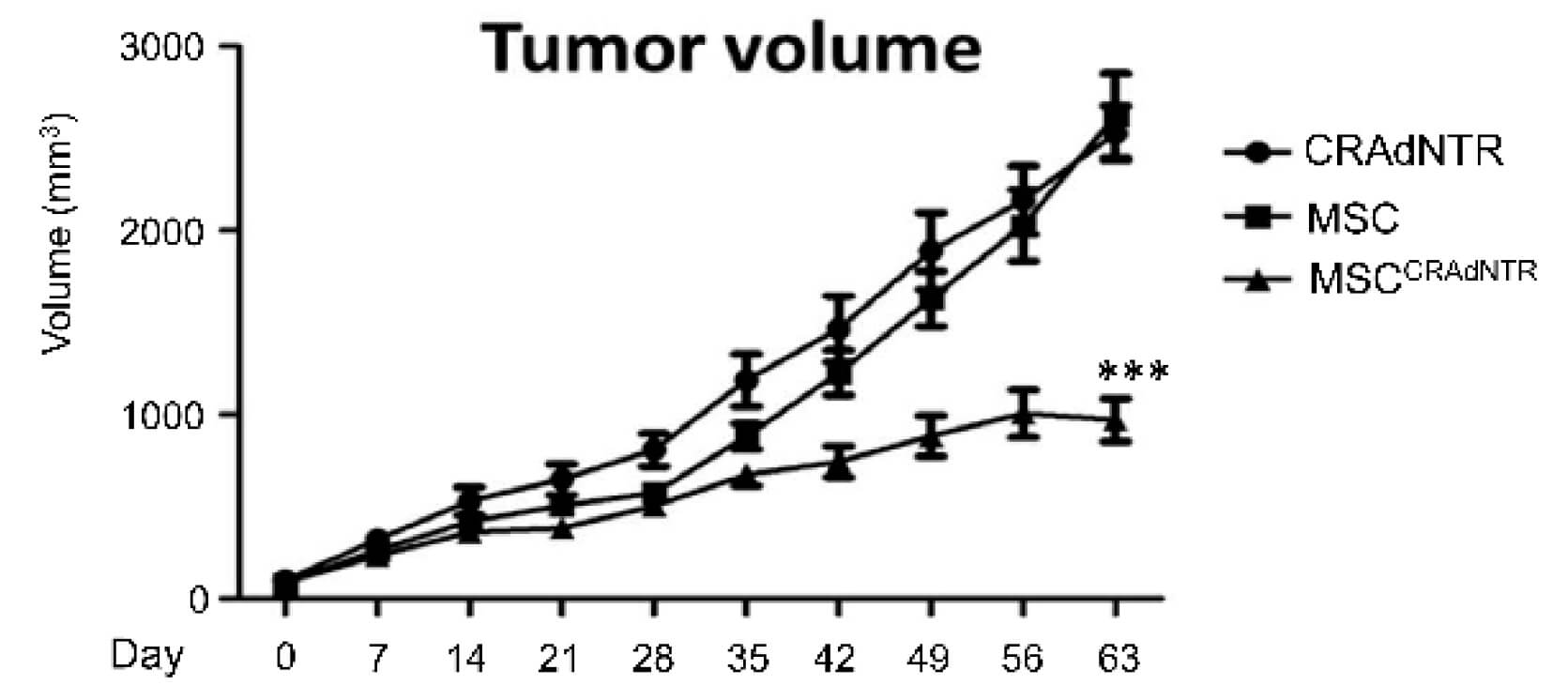
|
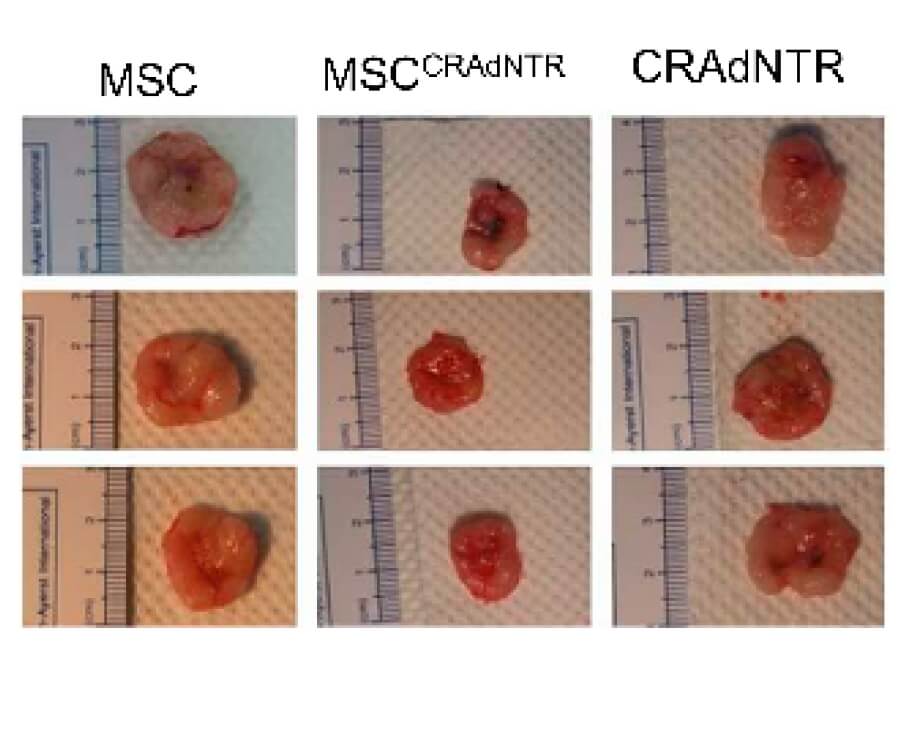
| Fig.4 HT29 colorectal cancer cells are used to establish a mouse model in vivo, and the tumors were photographed.2 | Fig.5 The tumor volume in the model mice is recorded every week and plotted as a line chart.2 |
FAQs
Q1: How does Creative Biolabs ensure the oncolytic virus targets only cancer cells and not healthy tissues?
A: Our oncolytic adenoviruses are genetically engineered to replicate only in cancer cells, using tumor-specific promoters or deleting genes unnecessary for cancer cell replication (e.g., RB/E2F pathway). Validated for safety and selectivity.
Q2: Will the prodrug interfere with the oncolytic virus's ability to replicate and spread within the tumor?
A: A key concern with armed oncolytic viruses is prodrug antiviral effects. Creative Biolabs selects enzyme/prodrug systems (e.g., NTR/DNBM) with no viral inhibition at relevant doses, and prodrugs even improve viral distribution/titre.
Q3: What kind of tumors can benefit from Creative Biolabs' Prodrugs-loaded Oncolytic Adenovirus service?
A: Our versatile technology applies to various solid tumors, with efficacy depending on tumor genetics, microenvironment, and enzyme/prodrug system. Preclinically effective in colorectal cancer models, we assess your tumor type and goals for optimal approaches.
Q4: How do you measure the effectiveness of the prodrugs-loaded oncolytic adenovirus in preclinical studies?
A: We use comprehensive preclinical assays: in vitro tests for cytotoxicity, viral replication, and bystander effects; in vivo studies monitoring tumor growth inhibition, viral distribution (via IHC), and prodrug activation (mass spectrometry). These assess efficacy and mechanism.
Q5: How does this approach compare to other gene therapy or oncolytic virus strategies?
A: Our Prodrugs-loaded Oncolytic Adenovirus merges tumor-selective oncolytics with localized prodrug activation for potent cytotoxicity. Unlike unarmed viruses, it adds direct chemotherapy and boosts viral spread. Compared to systemic chemo/gene therapy, it reduces off-target toxicity by confining drug action to tumors.
Creative Biolabs leads in advanced cancer therapies with our Prodrugs-loaded Oncolytic Adenovirus service. Combining viral engineering and localized prodrug activation, we enhance tumor cytotoxicity, improve viral spread, and reduce systemic toxicity. Trust our scientific excellence and client-focused approach for your next-gen oncology projects.
Contact Our Team to Discuss Your Project!
Related Sections
| Cytosine Deaminase loaded Oncolytic Adenovirus | Thymidine Kinase loaded Oncolytic Adenovirus |
| GM-CSF loaded Oncolytic Adenovirus | CD40L loaded Oncolytic Adenovirus |
| hNIS loaded Oncolytic Adenovirus | TNF-α loaded Oncolytic Adenovirus |
| IL-2 loaded Oncolytic Adenovirus | 41BBL loaded Oncolytic Adenovirus |
| PH20 Hyaluronidase loaded Oncolytic Adenovirus | Anti-CTLA4 loaded Oncolytic Adenovirus |
| Anti-PD1 loaded Oncolytic Adenovirus | IL-12 loaded Oncolytic Adenovirus |
| Decorin loaded Oncolytic Adenovirus | OX40L loaded Oncolytic Adenovirus |
| EGFR loaded Oncolytic Adenovirus | FRα loaded Oncolytic Adenovirus |
| FAP loaded Oncolytic Adenovirus | CD44v6 loaded Oncolytic Adenovirus |
References
- Markovic, Milica, Shimon Ben-Shabat, and Arik Dahan. "Computational simulations to guide enzyme-mediated prodrug activation." International journal of molecular sciences 21.10 (2020): 3621. DOI: 10.3390/ijms21103621. Distributed under Open Access license CC BY 4.0, without modification.
- Ho, Chun-Te, et al. "Combination of mesenchymal stem cell-delivered oncolytic virus with prodrug activation increases efficacy and safety of colorectal cancer therapy." Biomedicines 9.5 (2021): 548. DOI: 10.3390/biomedicines9050548. Distributed under Open Access license CC BY 4.0, the figures were cropped.
