Oncolytic Viruses in Multiple Myeloma Treatment
Introduction to Multiple Myeloma
Multiple myeloma (MM) is one of the commonest types of blood cancer which accounts for 1% of all cancers and approximately 10% of all hematologic malignancies. Like all cancers, factors that cause multiple myeloma are complex and poorly defined, including but not limited to exposure to organic chemicals, toxins, ionizing radiation. Genetic predisposition is also observed but the condition is not usually inherited. Non-specific symptoms of fatigue, loss of appetite, and weight loss are very common. Bone pain, back pain, and pathological fracture may also occur. Diagnosis of multiple myeloma is often completed through numerous methods, including but not limited to blood count, biochemistry analysis, paraprotein detection, and bone marrow examination.
Treatment for Multiple Myeloma
Currently, there is no cure for multiple myeloma. However, some treatments can help ease the pain, reduce complications, and slow the progression of the disease. Pharmacological therapy and autologous hematopoietic stem cell transplant (AHSCT) are usually used for the treatment of multiple myeloma. Three classes of drugs usually used include immunomodulatory agents, proteasome inhibitors and monoclonal antibodies. Some immunomodulatory drugs are thought to alter the tumor microenvironment in a manner that promotes MM cell killing by the immune system in addition to having anti-proliferative and anti-angiogenic effects. Proteasome inhibitors induce apoptotic death by preventing the normal turnover of cellular proteins. Cells that produce large quantities of protein, such as malignant plasma cells, are particularly sensitive to the effects of proteasome inhibition. Combination therapy with these drugs has significantly improved patient survival time and is now the preferred first-line treatment for newly diagnosed MM.
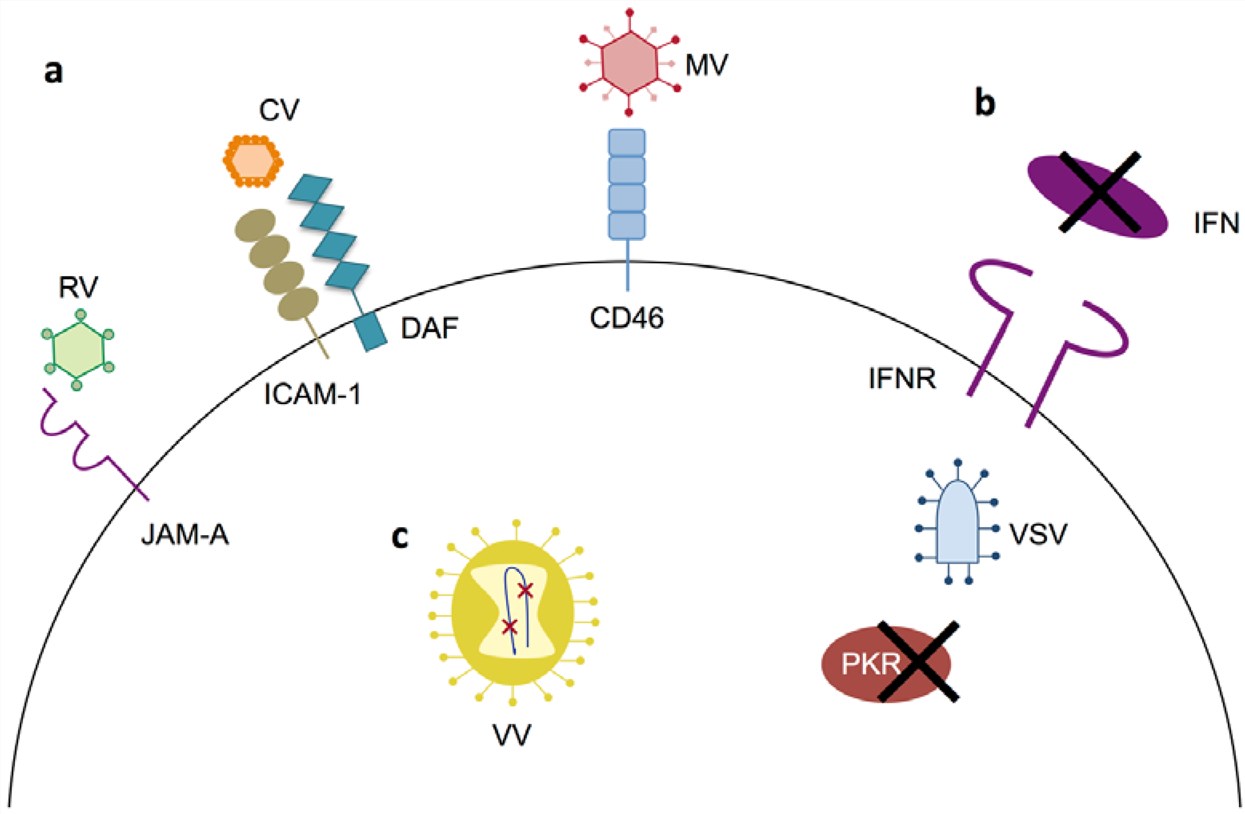 Fig.1 Primary mechanisms of tumor specificity for oncolytic virotherapy in multiple myeloma. (Calton, 2018)
Fig.1 Primary mechanisms of tumor specificity for oncolytic virotherapy in multiple myeloma. (Calton, 2018)
Oncolytic Viral Therapy for Multiple Myeloma
- Reovirus
- Measles Virus (MV)
- Vesicular Stomatitis Virus (VSV)
- Adenovirus
- Vaccinia Virus
Reoviruses (RV) belong to the Reoviridae family of viruses. The genome consists of 10 double-stranded RNA segments contained within two concentric protein shells. Three different RV serotypes have been identified and only the human type 3 Dearing strain is under development for oncolytic therapy. Preclinical studies have demonstrated that RV could kill MM cell lines and primary patient cells by inducing endoplasmic reticulum (ER) stress, apoptosis and autophagy and significantly reduce tumor burden in mouse xenograft models of MM. Combination treatment with RV results in a significantly greater reduction of disease than either single-agent treatment. Combining RV with the immunomodulatory agent also enhances MM cell killing. Currently, there are three active phases I trials combining RV with approved MM therapeutics. In addition, a clinical trial investigating RV with anti-PD-1 antibody therapy is also being developed based on promising preclinical data.
Measles virus (MV) is an enveloped negative-sense single-stranded RNA virus in the family Paramyxoviridae. Like many oncolytic viruses, MV has been shown specifically to infect both MM cell lines and CD138+ cells in MM-patient bone marrow. a preclinical study showed that the Edmonston strain of MV selectively replicates in and effectively kills myeloma cells from both established tissue culture lines and primary patient samples. Significant reductions in tumor volumes were also observed in mouse xenograft models following treatment with either intratumoral or intravenous injection of MV. Based on the preclinical results, a phase I/II trial was initiated to test the human sodium iodide symporter treatment in patients with recurrent or refractory myeloma in combination with or without an alkylating agent that is approved for myeloma therapy. Two other clinical trials for MV are currently active.
Vesicular stomatitis virus (VSV), a member of the family Rhabdoviridae, is an enveloped negative-sense single-stranded RNA virus. Commonly, VSV only infects cattle, horses. Human infections are limited to those in direct contact with infected animals. Therefore, VSV is an attractive candidate for oncolytic therapy. It has also been reported that VSV readily replicates in a variety of tumor cells which is a lack of PKR activation or type-I IFN production signaling. For oncolytic therapy development, a further attenuated strain by deleting methionine 51 in the VSV matrix protein (VSV∆51) has been created. This mutation ablates the virus' ability to block type-I IFN production, thus preventing infection of healthy cells, while maintaining a high degree of lytic activity in IFN-deficient tumor cells. The effectiveness of VSV in both in vitro and in vivo models of MM has been demonstrated by preclinical studies. Based on promising preclinical data, a phase I trial for VSV has been established. This trial will establish dosage and toxicities for VSV-hIFN-β-NIS in patients with relapsed/refractory MM.
Adenovirus (AdV) is a medium-sized (90-100 nm), non-enveloped, double-stranded DNA virus from the Adenoviridae family which has been used in a variety of therapeutic modalities, including lytically replicating oncolytic studies, vaccine studies, and gene-therapy studies. Actually, adenovirus includes a large number of distinct viral serotypes, many of which display highly distinct pathologies and infectious characteristics. Among these serotypes, AdV type 5 has been identified as the most commonly used oncolytic serotype for MM treatment. Unlike many oncolytic viruses, whose preferential infection of MM cells appears to be mediated by receptor specificity, the preferential killing of MM by AdV correlated better with replication kinetics than with viral adsorption. In order to improve this efficacy, several studies on the possibility of "arming" AdV to promote therapy have been established. Studies have shown that AdV armed with TRAIL can enhance the killing of MM cells in vitro and show therapeutic synergy with PI3K or proteasome inhibitors. In addition, AdV armed with CD40L has also displayed improved killing of MM cells both in vitro and in vivo.
The vaccinia virus (VacV) is a large (200-300 nm), enveloped, double-stranded DNA virus from the Poxviridae family. As an oncolytic agent, VacV has been well studied in the context of solid tumors in both preclinical and clinical settings. In particular, recombinant VacV encoding either GM-CSF or PSA has advanced to large Phase III clinical trials. However, the use of oncolytic VacV in a hematopoietic setting is much less well studied. This is likely because while VacV is extremely lytic, the virus is not naturally oncotropic and requires additional genetic modification to restrict viral replication to malignant cells. A variety of methods to enhance viral specificity have been attempted, such as deletion of the vgf gene in tk-/- clones and placement of essential viral genes under miRNA restriction. Nevertheless, further investigation into these approaches might be warranted.
Reference
- Calton, C.M.; et al. Oncolytic viruses for multiple myeloma therapy. Cancers. 2018, 10(6), p.198. Distributed under Open Access license CC BY 4.0, without modification.
