Oncolytic Viruses in Sarcoma Treatment
Introduction of Sarcomas
A sarcoma is an unusual type of cancer that is capable of affecting several human tissues, such as connective tissue, skin, bone, muscle, as well as tendons. In the past few years, many kinds of sarcomas, including but not limited to, Ewing sarcoma, liposarcoma, osteosarcoma, and epithelioid sarcoma have been identified and analyzed. As a rule, all the sarcomas can be divided into two main groups, soft tissue sarcoma, and bone sarcoma, based on the tissue and cell types of tumorigenesis. Bone pain, leg swelling, or a limp are common symptoms for sarcomas. Moreover, a series of factors has been proven to be associated with a high risk of developing sarcomas, such as long-term exposure to radiation and chemical reagent. Besides, recent reports have also revealed that heritable RB1 gene, EWS gene, and CDK4 have been regarded as biomarkers for soft tissue sarcoma. Nowadays, many methods have been generated for treating sarcomas. Surgery, radiation, and chemotherapy are effective strategies for reducing the local recurrence of soft tissue sarcomas. However, the treatment of sarcomas still has much room for improvement and no available therapy can inhibit the growth of sarcomas without causing any side effects.
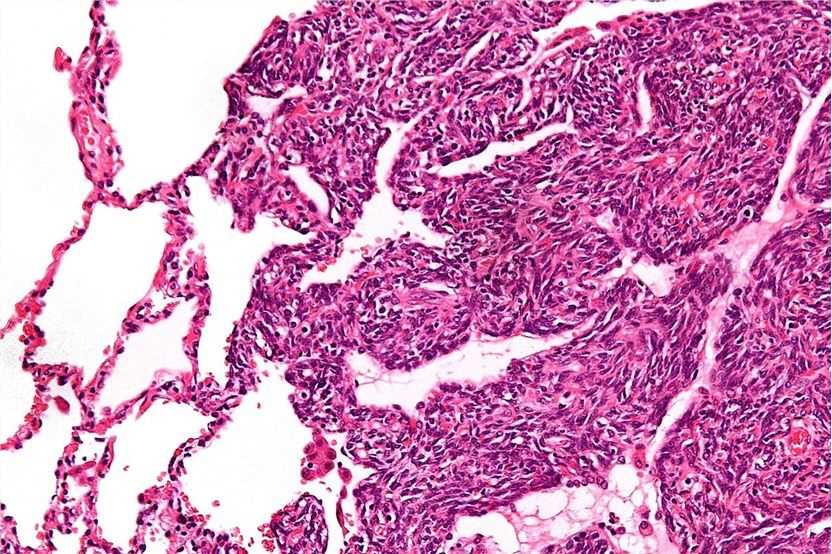 Fig.1 High magnification micrograph of a monophasic synovial sarcoma. Distributed under CC BY-SA 3.0, from Wiki, without modification.
Fig.1 High magnification micrograph of a monophasic synovial sarcoma. Distributed under CC BY-SA 3.0, from Wiki, without modification.
The Oncolytic Viruses in Sarcoma Treatment
Sarcomas are rare tumors that can occur in many parts of the human body. The most common sarcoma type is soft tissue sarcoma. In recent studies, many attempts have been made to improve overall survival for sarcomas patients. Many researchers have demonstrated that oncolytic viruses play an important role in selectively eliminating lysis cancer cells without damaging normal tissues in humans. Up to now, there is a battery of ways for oncolytic viruses to kill specific cancer cells, ranging from cytotoxic immune effects to virus-mediated cytotoxicity. As a consequence, oncolytic viruses have been considered as a novel therapy for the treatment of various cancers, especially for the advanced stage of sarcomas, in pre-clinical studies. The data have suggested that the genetically modified adenovirus has represented a promising method for triggering a strong immune response against sarcomas in mice models. Also, a recombinant and live-attenuated herpes simplex virus (HSV-1) used as an oncolytic virus has displayed sarcoma cell killing capacity in a series of animal models.
Furthermore, clinical trials of oncolytic virotherapy for sarcomas have been widely conducted in many ways and scales. For example, in phase I clinical trials, a randomized, double-blind, placebo-controlled study has been designed in patients with NY-ESO-1 positive synovial sarcoma following oncolytic virotherapy. In phase II clinical trials, the safety and side effects of oncolytic virus-based therapy will be further evaluated in treating patients with soft tissue sarcoma. Nowadays, several clinical trials are openly evaluating the use of reovirus, Newcastle disease virus, and Seneca valley virus, in soft and bone sarcomas. The results have indicated that these viruses are essential to infect specific tumor cells and antitumor efficacy. However, minimal immune adverse reactions, such as fever, have been observed in patients receiving oncolytic virotherapy. Therefore, new strategies still need to be developed to increase safety and reduce the potential for side effects caused by the application of live viruses. As a result, the use of oncolytic virotherapy in conjunction with conventional therapies have been broadly studied in recent trials. It provides a unique opportunity for the use of oncolytic virus-based combined therapy as a treatment for sarcoma in the future.
