Oncolytic Viruses in Gastric Cancer Treatment
Oncolytic virus therapy (OVT) has recently been the focus of extensive oncology research and shown success in preclinical and clinical testing as a novel cancer treatment modality with high safety profiles and low off-target toxicities. OVT is also called oncolytic virotherapy, viral therapy, and virotherapy whose antitumor action is based on the destructive nature of viruses to kill tumor cells in cancer patients. Therefore, the ultimate aim of OVT is to design a virus which can effectively replicate within the host, specifically target and lyse tumor cells and induce robust, long-lasting tumor-specific immunity.
Gastric Cancer
Gastric cancer (GC), also called stomach cancer, is the second leading cause of death from cancer worldwide. This deadly disease is characterized by a growth of cancerous cells within the lining of the stomach, but shows heterogeneity with epidemiologic and histopathologic differences across countries. GC is difficult to diagnose because most people typically don't show symptoms in the earlier stages, resulting in late diagnosis, suboptimal therapies and poor survival rate.
- Symptoms of GC
- Risk Factors of GC
- Diagnosis of GC
- Treatment of GC
Usually, there are typically no early signs or symptoms of GC. Advanced GC shows some common symptoms, including nausea and vomiting, frequent heartburn, loss of appetite accompanied by sudden weight loss, constant bloating, early satiety, bloody stools, jaundice, excessive fatigue, stomach pain, etc.
There is no exact reason what makes cancer cells start growing in the stomach. But there are some factors that might increase your risk for the disease, including lymphoma, H. pylori bacterial infections, tumors in other parts of the digestive system, stomach polyps, gastritis, smoking, being overweight or obese, a diet high in smoked, pickled, or salty foods, etc.
physical exam to check for any abnormalities, including H. pylori bacterial test; blood tests; upper gastrointestinal endoscopy; a biopsy; imaging tests, such as CT scans and X-rays.
Multidisciplinary approach for the treatment of GC is necessary, including chemotherapy, radiation therapy, surgery, and immunotherapy. Especially, oncolytic virus therapy has become a promising treatment option for GC.
 Fig.1 Gastric cancer.
Fig.1 Gastric cancer.
Oncolytic Viruses in GC
There are a number of viruses that can be used to play oncolytic functions in GC therapy, mainly including adenovirus (Ad), vaccinia virus (VACV or VV), herpes simplex virus (HSV). They present either naturally tumor-selective or can be modified to specifically target and eliminate tumor cells. During oncolytic virotherapy, specificity is imperative in order to reduce the side effects.
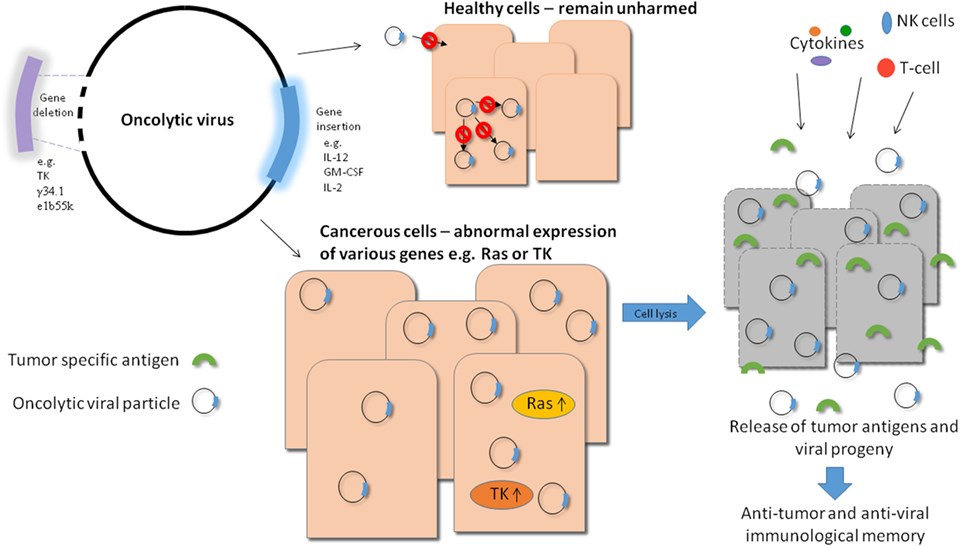 Fig.2 Overview of oncolytic viral therapy. (Howells, 2017)
Fig.2 Overview of oncolytic viral therapy. (Howells, 2017)
- Adenovirus
Ad, belonging to Adenoviridae family, is a non-enveloped, double-stranded DNA virus with an icosahedral capsid encompassing a linear duplex genome of ~36 kb. It is one of the widely studied viruses in oncolytic therapy, amongst, Ad5 is the most commonly used. China approved the first recombinant adenovirus for nasopharyngeal cancers in November 2006. In GC virotherapy, Ads are being developed to maximize tumor-cell killing potency while minimizing toxicities.
- Vaccinia Virus
VACV is a double-stranded DNA virus of the Poxviridae family. It is a naturally oncolytic virus which was found to have a natural tropism for tumor cells due to its sensitivity to type I interferon. The strains Lister, Wyeth, and Western Reserve are the most used in OV research, both of which can incorporate large amounts of foreign DNA without reducing their replication efficiency. Moreover, VACV entry does not have a specific receptor which makes it a potential candidate for the treatment of all tumor types.
- Herpes Simplex Virus
HSV is a double-stranded DNA virus belonging to the Herpesviridae family. HSV-1 is the first strain in which TK gene mutation was engineered and showed several benefits like: (a) large genome and power to infiltrate in the tumor, (b) easy access to manipulation with the flexibility to insert multiple transgenes, (c) infecting majority of the malignant cell types with quick replication in the infected cells.
Oncolytic Viruses Modification
The specificity of OVs often determines the success of therapy. There are various ways have been developed to improve the specificity of OVs, such as tumor-specific promoters (TSPs) for conditionally replicating, insertion and deletion of specific genes to improve recognition efficacy. Taking advantage of pathways that are upregulated in tumor cells and not healthy cells, we can engineer a virus that relies on such a pathway for successful infection thereby rendering the virus incapable of infecting healthy tissue.
OVs are being developed to address various malignancies (such as GC) with improved specificity and efficacy in cancer therapeutics.
Reference
- Howells, A.; et al. Oncolytic viruses-interaction of virus and Tumor Cells in the Battle to eliminate Cancer. Frontiers in oncology. 2017, 7: 195. Distributed under CC BY 4.0, without modification.
