Copper(I)-Catalyzed Alkyne-Azide Cycloaddition (CuAAC)
What is Copper(I)-Catalyzed Alkyne-Azide Cycloaddition (CuAAC)?
CuAAC is a variant of the Huisgen 1,3-dipolar cycloaddition reaction, first proposed in 2002 by American chemist Karl Barry Sharpless. It has rapidly emerged as one of the most classic examples of "Click Chemistry". The CuAAC reaction is based on the copper(I)-catalyzed cycloaddition between azides (R-N3) and alkynes (R'-C≡C-H), resulting in the formation of 1,2,3-triazole compounds.
Mechanism of CuAAC
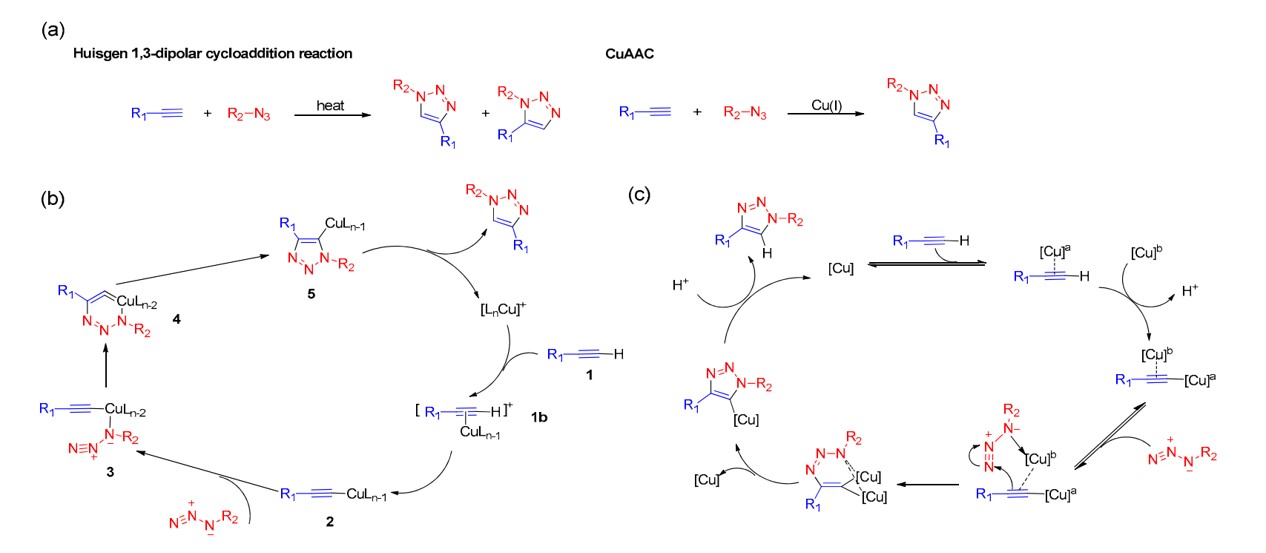 Fig.1 CuAAC reaction and mechanism.1
Fig.1 CuAAC reaction and mechanism.1
The reaction mechanism involves the formation of a highly reactive copper(I) acetylide complex through the interaction of a copper catalyst with terminal alkynes. Subsequently, the azide moiety performs a nucleophilic attack on this complex, leading to the formation of a copper heterocycle, which undergoes rearrangement to yield a copper(I) triazole intermediate. The intermediate then undergoes protonation, facilitating the formation of the final 1,4-substituted triazole product. Early mechanistic studies of the CuAAC reaction posited that the monomeric copper(I) acetylide complex acted as an intermediate within the catalytic cycle. However, further monitoring of the reaction process suggests the involvement of binuclear or multinuclear copper(I) intermediates.
Features of CuAAC
- Fast reaction rates comparable to cysteine-maleimide reactions.
- Robust activity across a wide range of pH levels (pH 4-12) and temperatures.
- High reaction specificity, insensitivity to water, and relative inertness towards biological compounds.
- The reaction products can be obtained through straightforward methods such as filtration and extraction, eliminating the need for more complex purification techniques like column chromatography or recrystallization.
- The strong nucleophilic/electrophilic properties of azides/alkynes enable these compounds to be easily incorporated into the structures of organic molecules, facilitating diverse synthetic applications.
Disadvantages of CuAAC
- Copper(I) catalysts exhibit toxicity and readily chelate with natural amino acid residues, compromising protein structure and function while also promoting the generation of reactive oxygen species (ROS).
- Under organic conditions or in buffer environments, reactions often yield by-products, including diazide, 5-hydroxytriazole, bis-triazole, etc.
Development of CuAAC
![]()
The use of water-soluble ligands, such as tris(3-hydroxypropyltriazolylmethyl)amine (THPTA), can effectively reduce the toxicity of copper(I) catalysts. THPTA significantly enhances the stability of Cu+ in solution, thereby decreasing the necessary amount of metal catalyst and improving reaction efficiency. Additionally, the triazole structure in THPTA exhibits antioxidant properties, serving as a reactive oxygen species (ROS) scavenger to protect reactants from oxidative degradation. These ligands greatly expand the applicability of CuAAC, enabling rapid and efficient reactions in aqueous solutions, at low concentrations, and within physiological conditions.
![]()
Another strategy involves utilizing copper-chelating azides designed through the concept of chelation-assisted metal catalysis to accelerate reactions, significantly reducing the required amount of Cu(I) catalyst. This approach is considered to enhance the electrophilicity of the azide group, thereby facilitating the formation of metallacycle intermediates.
Bioorthogonal Applications of CuAAC
CuAAC has emerged as a prominent bioorthogonal reaction extensively utilized for the labeling and detection of various biomolecules, including proteins, nucleic acids, carbohydrates, and lipids. An example of protein labeling involves the selective tagging of protein tyrosine phosphatase (PTP) in live cells using redox probes containing alkynes, followed by monitoring PTP through CuAAC facilitated by 3-(4-((Bis((1-(tert-butyl)-1H-1,2,3-triazol-4-yl)methyl)amino)methyl)-1H-1,2,3-triazol-1-yl)propan-1-ol (BTTP). Additionally, CuAAC has been utilized for the linkage of hetero or homo multimeric proteins, enabling site-specific protein-protein conjugation. For instance, a cell-free protein synthesis system (CFPS) can incorporate unnatural amino acids (UAAs) equipped with alkyne and azide groups into two distinct proteins, which can then undergo direct coupling via CuAAC. This method circumvents the need for chemical spacer reagents, preserving the activity of the conjugated heteromeric proteins.
References
- Li, Li, and Zhiyuan Zhang. "Development and applications of the copper-catalyzed azide-alkyne cycloaddition (CuAAC) as a bioorthogonal reaction." Molecules 21.10 (2016): 1393. Distributed under Open Access license CC BY 4.0, without modification.
Related products
