Strain-Promoted Azide-Alkyne Cycloaddition (SPAAC)
What is Strain-Promoted Azide-Alkyne Cycloaddition?
The cycloaddition of azides and alkynes stands out as one of the most efficient, quintessential, and practically valuable reactions in the field of click chemistry. Unlike the copper-catalyzed azide-alkyne cycloaddition (CuAAC), which necessitates the use of a copper catalyst, SPAAC leverages the inherent high ring strain of cyclic alkynes to facilitate a chemically regioselective click reaction without any additional catalytic assistance. This unique characteristic significantly broadens the applications of click chemistry in diverse fields such as bioconjugation and materials science.
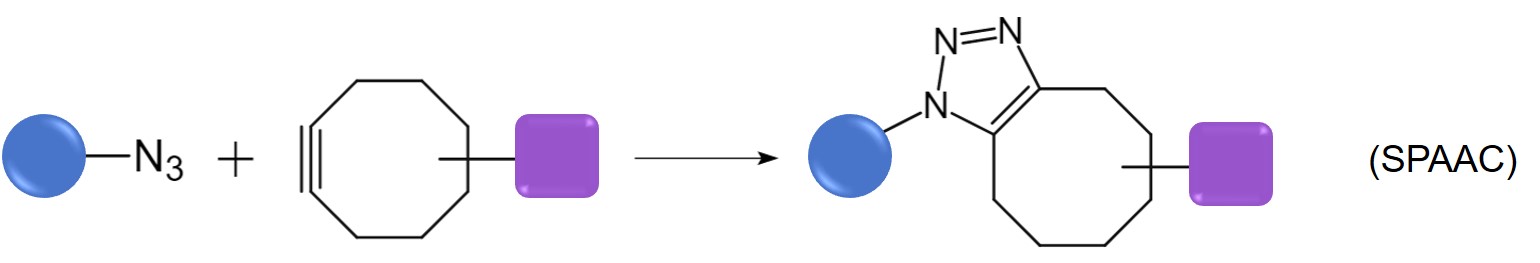
Advantages of SPAAC over classic AuCCA
- SPAAC does not require the presence of copper, thus mitigating the cytotoxicity associated with copper ions and allowing the reaction to proceed in vivo.
- Cycloalkynes used in SPAAC are less prone to side reactions than terminal alkynes.
- SPAAC exhibits remarkable stability and reactivity across a wide range of environmental conditions, demonstrating insensitivity to factors such as acidity, alkalinity, and the presence of water.
Disadvantages of SPAAC
- The traditional cyclooctyne reaction is characterized by a slow reaction rate, often necessitating high reagent concentrations and extended incubation times to achieve completion. This prolonged process typically results in a relatively low final signal.
- The reaction lacks selectivity for 1,4 or 1,5 additions.
Development of SPAAC
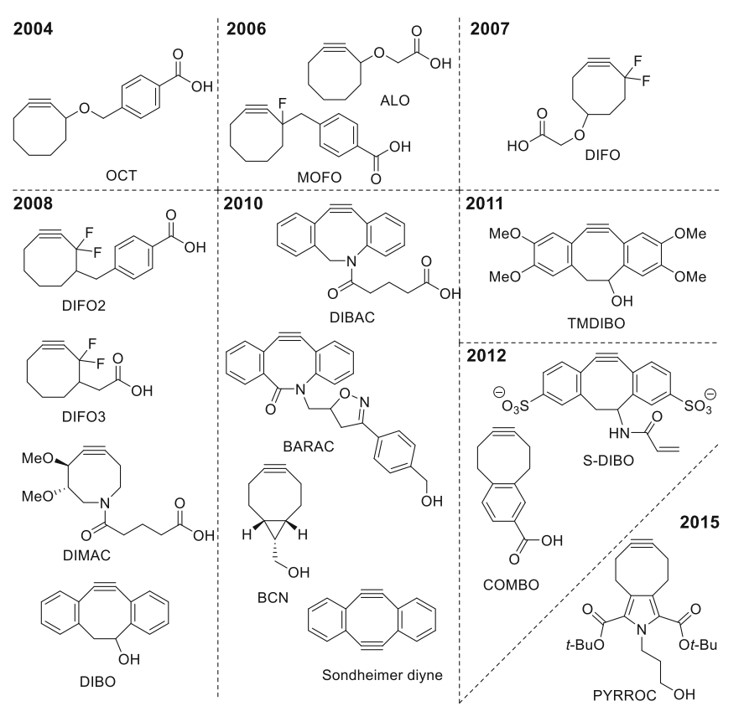 Fig.1 Functionalized cyclooctynes suitable for conjugation reactions.1
Fig.1 Functionalized cyclooctynes suitable for conjugation reactions.1
The reaction rate of SPAAC is directly influenced by the structure of cyclooctynes, which can be accelerated through modifications of these compounds. However, excessively high reaction rate constants can lead to instability in the cyclooctyne substrates, limiting their practical applications. Currently, the most widely used substrates in SPAAC reactions are the highly reactive yet stable bicyclononyne (BCN) and dibenzocyclooctyne (DBCO, also known as dibenzoazacyclooctyne (DIABC) or aza-dibenzocyclooctyne (ADIBO)). BCN, developed by Dommerholt et al., features a small volume and a straightforward synthesis route, allowing it to participate stably in SPAAC reactions. Meanwhile, DBCO demonstrates even greater reactivity and stability, which facilitates linkage with other functional groups, making it a favored reagent in click chemistry.
Applications of SPAAC
SPAAC reaction has gained prominence across various fields such as biomedicine and chemical materials, thanks to its simplicity, efficiency, mild reaction conditions, and bioorthogonal nature.
![]()
Molecular imaging
SPAAC facilitates the development of innovative imaging probes by enabling the labeling of active molecules or biomolecules, such as proteins and nucleic acids, allowing for real-time tracking of their dynamics within living organisms. This technology is instrumental not only in cellular protein imaging but also in investigating drug-target interactions.
![]()
Targeted drug delivery
In therapeutic applications, the characteristics of SPAAC prove highly beneficial for the synthesis of polymers in drug carrier development and the site-specific modification of bioligands. For example, by inducing the production of azide groups in tumor cells through the metabolic precursor Ac4ManNAz, researchers can subsequently introduce drug carriers modified with handles such as DBCO or BCN. This approach enables effective tumor targeting of the drug through SPAAC reactions in vivo.
![]()
Macromolecule derivatization
The SPAAC reaction is also employed for the chemical modification of macromolecules, like proteins, nucleic acids, nanoparticles, and polymers. These modifications can alter the physicochemical properties of the molecules, enhance their stability, improve biocompatibility, or confer new biological functions. Such derivatization plays a significant role in applications such as vaccine development, antibody engineering, and the preparation of biomaterials.
Reference
- Dommerholt, Jan, Floris PJT Rutjes, and Floris L. van Delft. "Strain-promoted 1, 3-dipolar cycloaddition of cycloalkynes and organic azides." Cycloadditions in Bioorthogonal Chemistry (2016): 57-76. Distributed under Open Access license CC BY 4.0, without modification.
Related products
