Staudinger Ligation
What is Staudinger Ligation?
In 1919, chemists Staudinger and Meyer discovered that organic azide could react with trialkylphosphine or triphenylphosphine (TPP) to form an iminophosphorane intermediate. In an aqueous environment, this intermediate undergoes hydrolysis, yielding primary amine and triphenylphosphine oxide (TPPO). This reaction became known as the Staudinger reaction. In 2000, Professor Bertozzi and her team recognized the potential of this reaction in bioconjugation and refined it by designing a chemical linkage that selectively generates an amide bond between reactants, successfully transforming it into Staudinger ligation.
Mechanism of Staudinger Ligation
Staudinger Ligation is mechanistically similar to the Staudinger reaction, initiated by the nucleophilic attack of TPP on the terminal nitrogen atom of an azide. This reaction results in the release of nitrogen, resulting in the formation of an iminophosphorane intermediate. Subsequently, this intermediate undergoes cyclization to yield a cyclic oxazaphosphetane intermediate, which can be hydrolyzed to obtain the final product, characterized by an amide linkage.
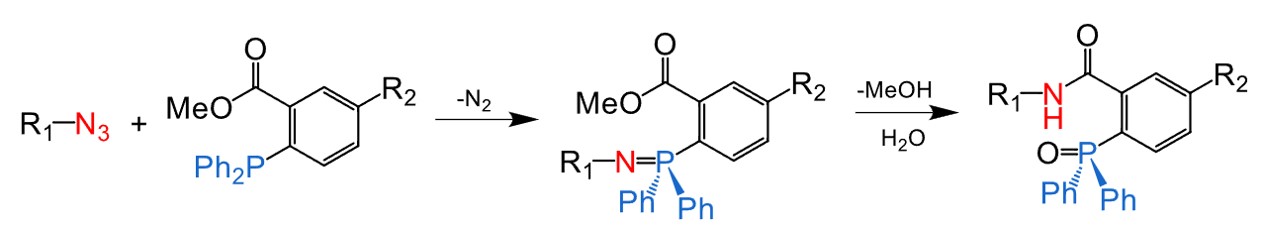 Fig.1 The Staudinger ligation.1
Fig.1 The Staudinger ligation.1
Traceless Staudinger Ligation
In 2000, the Raines and Bertozzi groups simultaneously reported a novel Staudinger ligation known as traceless Staudinger ligation. This reaction represents an advancement over the original Staudinger ligation, with its "traceless" characteristic achieved by generating an amide bond while simultaneously removing TPPO during the hydrolysis step. Traceless Staudinger ligation has proven to be a powerful tool for peptide conjugation, effectively forming natural amide bonds and serving as a viable alternative to traditional chemical linkages.
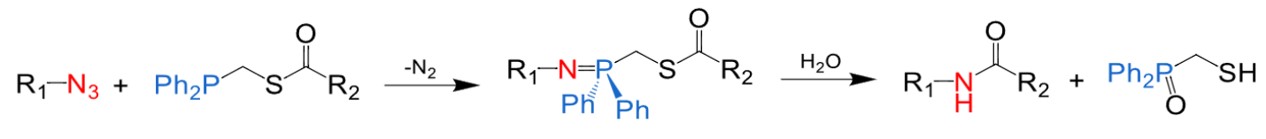 Fig.2 The traceless Staudinger ligation.1
Fig.2 The traceless Staudinger ligation.1
Applications of Staudinger Ligation
Staudinger ligation exhibits key characteristics of bioorthogonal chemistry, including excellent chemical selectivity, biocompatibility, absence of potential cytotoxicity, rapid reaction kinetics, high yields, minimal side products, and readily available starting materials. These attributes have facilitated its widespread application in chemical biology, especially in biomolecular labeling. Furthermore, the reaction is extensively utilized in the innovative design of drug delivery systems, the development of biosensors, and the engineering of cell surfaces, thereby advancing biomedical research and related technologies.
 Labeling Biomolecules
Labeling Biomolecules
Staudinger ligation is commonly employed for labeling small molecules onto biomolecules. This bioconjugation method necessitates the presence of phosphines or azides for the reaction to proceed within biological macromolecules. The molecular size and stability of azides under physiological conditions make them a preferred choice as reactants. Additionally, some azides can be readily conjugated to glycans, proteins, lipids, DNA, or RNA through chemical methods, such as diazo transfer, or via fundamental biosynthetic pathways.
 Drug Delivery System Development
Drug Delivery System Development
Liposomes play a crucial role in the encapsulation of therapeutic agents and drug delivery. In recent years, various chemoselective coupling methods have been employed to functionalize the surface of liposomes, including imine or hydrazone linkage, thiol-maleimide coupling, copper(I)-catalyzed alkyne-azide cycloaddition (CuAAC), and Staudinger ligation. Among these methods, Staudinger ligation stands out for its ability to achieve specific labeling of lipids under mild conditions—room temperature, aqueous solutions, and without the need for catalysts. This method is compatible with many unprotected functional groups of biomacromolecules, thereby demonstrating broader applicability in the development and delivery of functionalized liposomes.
 Biosensor Development
Biosensor Development
The leaving groups generated during Staudinger ligation can be strategically designed for specific biological applications, such as imparting chromogenic or fluorescent properties. As a result, Staudinger ligation has gained significant traction in the development of fluorescent biosensors, offering a rapid and convenient approach for detecting nucleic acids and small molecular substances.
Reference
- Bird, Robert E., et al. "Bioorthogonal chemistry and its applications." Bioconjugate Chemistry 32.12 (2021): 2457-2479. Distributed under Open Access license CC BY 4.0, without modification.
