The decay-accelerating factor DAF (CD55) physiologically serves as an inhibitor of the complement system. With the development of research and technology, DAF seems to dispose of several different functions reaching far beyond its immunological role, such as decrease of complement mediated tumor cell lysis, promotion of tumorigenesis, autocrine loops for cell rescue and evasion of apoptosis. Now Creative Biolabs has successfully developed a series of DAF-realted services and effective Antibody, DAF-realted protein, Arrar kits products.
Structure and Function of DAF
DAF is a 70 kDa glycosyl phosphatidylinositol (GPI) anchored membrane protein. The gene encoding for DAF has been identified on the long arm of chromosome 1q32 within a stretch of genes encoding for regulators of complement activation (RCA). The structure of the DAF protein is shown in Figure 1. Every DAF molecule contains four units for the control of complement activation following each other and that are designated as complement control protein repeats (CCP) 1 to 4, followed by a 97-aa O-glycosylation-rich region. Most of the DAF isoforms are linked to the cell membrane by the GPI anchor. Even though the calculated molecular weight (MW) of the DAF protein is 43 kDa, the observed MW of mature DAF varies between 50 to 100 kDa depending on the cell type. Different sizes of DAF might be caused by different glycosylation patterns or alternative splicing.
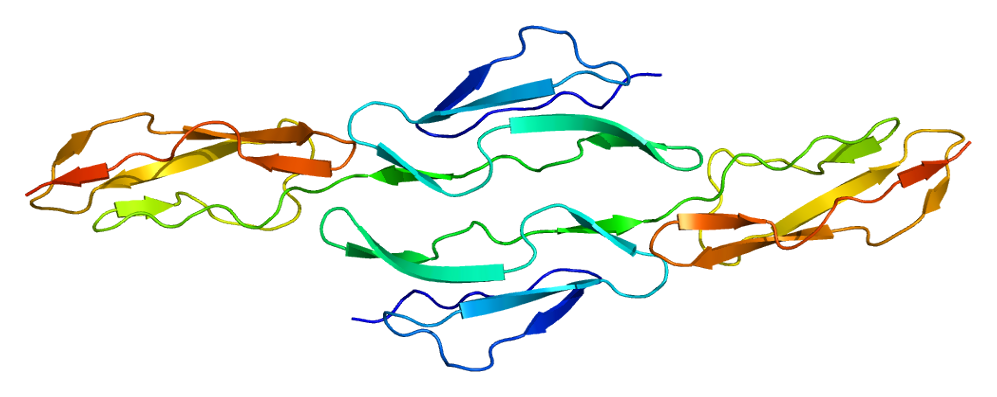
Fig. 1 Structure of DAF.1
DAF is present on various cell types such as erythrocytes, lymphocytes, granulocytes, endothelium, and epithelium. A primary function of DAF is to protect the host cell from autologous complement attack. The best-known function of DAF is regulating the complement system activity by inhibiting C3 convertases (C4b2a and C3bBb) and C5 convertases (C4b2a3b and C3bBb3b) on the cell surface. DAF interacts with the convertases and destabilizes by inducing rapid dissociation of a catalytic subunit C2a or Bb. The inhibitory action of DAF is restricted to C3/C5 convertases bound directly to host cells. DAF does not inhibit normal complement activation on targets, such as microorganisms and immune complexes. Besides, DAF also appears to inhibit natural killer cells. Moreover, DAF is known as a receptor for certain viruses and microorganisms and as a ligand of the CD97 receptor. Lastly, recent studies have suggested a direct inhibitory role in T cell and macrophage activation.
DAF Deficiency
DAF deficiency is linked to a lot of diseases including paroxysmal nocturnal haemoglobinuria (PNH), autoimmune diseases such as rheumatoid arthritis, systemic sclerosis, vitiligo, psoriasis as well as in some gastrointestinal diseases like ulcerative colitis. In xenotransplantation, DAF is used for the prevention of hyperacute and acute graft rejection. Inherited deficiency of DAF is uncommon. Since the Cromer blood group system is located on the DAF molecule, patients with genetically determined DAF deficiency are negative for all Cromer antigens, and possess the Inab phenotype. Besides the inherited deficiency, an acquired deficiency also exists. Patients with the acquired deficiency have a clonal red cell population lacking GPI anchored proteins, including protectin (CD59) and DAF, and the clinical disorder paroxysmal nocturnal hemoglobinuria (PNH).
Recent years, expression of the DAF in human malignancies has become an interesting subject of cancer research. Based on the frequent expression of DAF in human malignancies, DAF might be a useful protein for diagnostics. Thereby, studies applying monoclonal anti-DAF antibodies and anti-DAF vaccination for a targeted therapy have been enrolled recently. For the future, it appears interesting to reveal more details about the role of DAF. If additional help is needed, please directly contact us and consult our technical supports for more details.
Reference
-
By Emw - Own work, CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=8764174
Related Product
For Research Use Only.
Related Sections:

