Regulation of complement activation is primarily mediated by the regulators of complement activation (RCA) family proteins which are formed by tandemly repeating complement control protein (CCP) domains. Mutations and polymorphisms in RCA proteins are linked to various diseases such as age-related macular degeneration, dense deposit disease, atypical haemolytic uraemic syndrome. Creative Biolabs provides a wide range of complement regulator development services and related-products towards RCA proteins.
RCA Family Introduction
In humans, RCA proteins cluster on the chromosome 1q32. A characteristic feature of the RCA proteins is the presence of CCP modules, which are linked by short linkers of 3-8 amino acids (aa). These CCP modules are composed of about 60-70 aa with four invariant cysteines forming disulfide bonds between CI-CIII and CII-CIV. The number of CCP modules in human RCA proteins vary from 4 to 59. Deletion mutagenesis showed that a minimum of 3-4 successive CCP domains in RCA proteins contribute to complement regulation and cell protection from complement-mediated damage.
The notable members of this family include decay-accelerating factor (DAF/CD55), factor H, membrane cofactor protein (MCP/ CD46), and C4b binding protein (C4BP). DAF and MCP are expressed on cell membranes, where they provide immediate protection to host cells. The soluble regulator factor H stops complement activation in the fluid phase and discriminates self from nonself cells and matrix material by binding host molecular patterns.
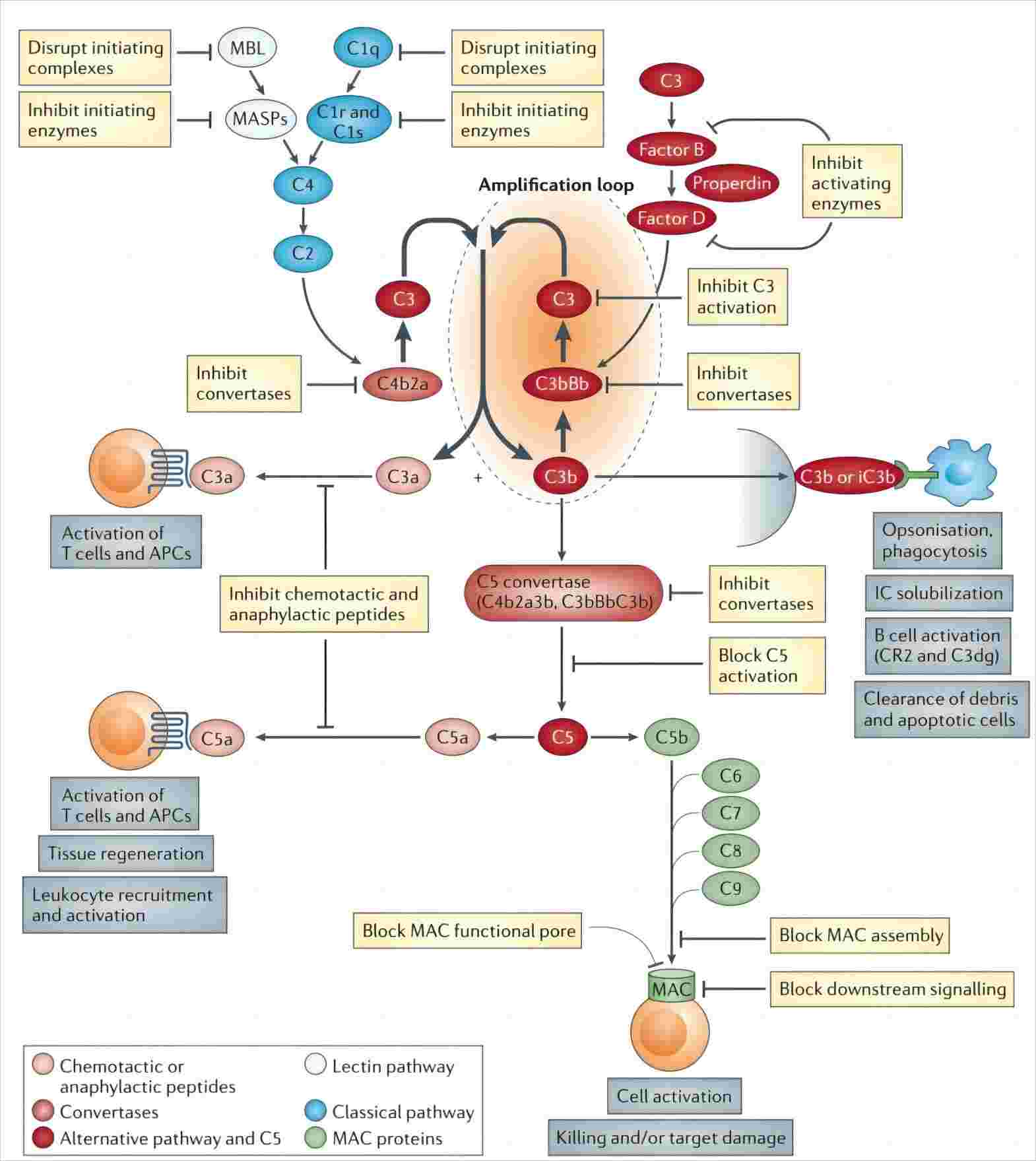
Fig 1. Schematic diagram of complement regulation.1
-
Surface-bound regulators: DAF, MCP
DAF, a 70 kDa glycoprotein, is a widely expressed glycosylphosphatidylinositol (GPI) anchor membrane protein that binds to and dissociates both the alternative (C3bBb) and the classical (C4b2a) pathway C3/C5 convertase enzymes, thus limiting the formation of the C5 convertase and ultimately the formation of the membrane attack complex (MAC). Because CD55 is a membrane protein, it only inhibits complement activation on cells that express it.
MCP is a transmembrane cell surface protein that also accelerates the decay of C3 convertases, protecting cells from autologous complement mediated lysis. MCP is expressed on most cell types but not on erythrocytes. In conjunction with soluble factor I, MCP also inactivates C3b to iC3b, thereby preventing reformation of the C3 convertase. MCP also acts as a receptor for several pathogens such as measles and adenoviruses. Additionally, MCP is a powerful regulator of T cell-mediated immunity.
-
Soluble regulators in the alternative pathway: Factor H
Factor H is a 150 kDa single-chain plasma glycoprotein that is an important regulator of the alternative pathway. Specifically, factor H regulates complement activation by three distinct mechanisms, acting as a cofactor for factor I, inhibiting the interaction between C3b and factor B thereby blocking the formation of C3bBb, contributing to the dissociation of C3 convertase. Factor H can also facilitate the decay of already formed C3bBb C3-convertase by displacing bound Bb from C3b.
Functional Mechanism of RCA Family
Complement regulation is important for protecting the body’s own cells from homologous complement. This function is primarily mediated by the regulators of RCA proteins via two functional mechanisms, decay-accelerating activity (DAA) and cofactor activity (CFA). In DAA, the RCA protein binds to the convertase and irreversibly dissociates it into its subunits. In CFA, the RCA protein binds to the noncatalytic subunit of the convertase (C3b/C4b) and recruits serine protease factor I to cleave and inactivate it, thus ending its ability to form C3 convertase.
Reference
-
Morgan, B. Paul, and Claire L. Harris. "Complement, a target for therapy in inflammatory and degenerative diseases." Nature reviews Drug discovery 14.12 (2015): 857-877.
Related Product
For Research Use Only.
Related Sections:

